Help your patients strengthen their bones
iBoneAcademy is your source for scientific information on osteoporosis. Here you can access disease state education slides and video presentations to learn more about fractures and osteoporosis management.
Globally, approximately 1 in 3 women ≥ 65 years of age who experience vertebral fractures are not diagnosed.1 Learn why screening plays an essential role in helping patients strengthen bones and what you as a physician can do to help prevent, diagnose, and manage this serious condition.
Osteoporosis is underdiagnosed and undertreated in patients who sustain a fragility fracture.3,4 Watch this video to learn how fragility fractures could be a warning sign for osteoporosis.2
#usa-785-80660Osteoporosis is a common bone disease characterized by low bone mass and structural deterioration of bone tissue, leading to bone fragility and an increased risk of fractures.5 Watch this video to review the pathophysiology, consequences, management tips, and clinical guidelines of osteoporosis.
#usa-785-81419The rate of osteoporosis-related fractures has increased, but the rate of diagnosis of osteoporosis has decreased.6-8 In this video, learn about recent trends in osteoporosis that could help you identify and treat patients with osteoporosis.
#usa-785-81693Using the patient education chapter book, learn about ways to reduce the risk of osteoporosis-related fractures, including at home steps that can be taken to care for your bones after an osteoporosis-related fracture, and how caregivers can help patients.
Osteoporosis affects 200 million women, worldwide.9 Learn about the incidence and treatment rates of fragility fractures in this population.
#usa-785-81932Fragility fractures result in more hospitalizations than breast cancer, stroke, or myocardial infarction.10 Explore its significant clinical and personal burden on patients with osteoporosis.
#usa-785-81933Role of a primary care practitioner in post-
fracture care
Proper diagnosis can help prevent future
fractures
associated with progressive bone
loss and aging11,12
1. Delmas PD, et al. J Bone Miner Res. 2005;20:557-563. 2. Watts NB, et al. Endocr Pract. 2010;16(suppl 3):1-37. 3. International Osteoporosis Foundation. www.iofbonehealth.org/facts-statistics. Accessed August 22, 2023. 4. Yusuf AA, et al. Arch Osteoporos. 2016;11:31. 5. WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1-129. 6. Wright NC, et al. J Bone Miner Res. 2014;29(11):2520-2526.2014;29(11):2520-2526. 7. Lewiecki EM, et al. JBMR Plus. 2019;3(9):e10192. 8. Lewiecki EM, et al. Hip fractures and declining DXA testing: at a breaking point? Presented at: American Society for Bone and Mineral Research Annual Meeting. September 16-19, 2016; Atlanta, GA. Abstract 1077. 9. International Osteoporosis Foundation. https://www.osteoporosis.foundation/healthprofessionals/ about-osteoporosis/epidemiology. Accessed August 22, 2023. 10. Singer A, et al. Mayo Clin Proc. 2015;90:53-62. 11. National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2014. 12. Siris ES, et al. Osteoporos Int. 2014;25:1439-1443.
These materials are provided for educational and non-commercial purposes only. All materials provided herein are licensed for use only under the Creative Common Attribution-Non-Commercial-No Derivatives 4.0 International Public License linked here.
This transcript is provided for your convenience and is qualified by the full video in which the spoken content appears. Please review the full video along with the transcript.
In general, there is a lack of public awareness about osteoporosis.1
and a misconception that it is an unavoidable part of aging.2
Osteoporosis is more prevalent than you think. Approximately 200 million women worldwide are affected by osteoporosis.3
and worldwide 1 in 3 women over 50 years of age will suffer a fragility fracture.4
and fewer than 1 in 3 may be treated, even after experiencing a fragility fracture.5
References:
1. Harvey NC, McCloskey EV, Mitchell PJ, et al. Mind the
(treatment) gap: a global perspective on
current and future strategies for prevention of fragility
fractures. Osteoporos Int. 2017;28:1507-
1529.
2. Feldstein AC, Schneider J, Smith DH, et al. Harnessing
stakeholder perspectives to improve the
care of osteoporosis after a fracture. Osteoporos Int.
2008;19:1527-1540.
3. International Osteoporosis Foundation.
Epidemiology.
https://www.osteoporosis.foundation/health-professionals/aboutosteoporosis/
epidemiology. Accessed August 18, 2022.
4. International Osteoporosis Foundation. Epidemiology of
osteoporosis and fragility fractures.
https://www.osteoporosis.foundation/facts-statistics/epidemiology-of-osteoporosis-andfragility-
fractures. Accessed August 18, 2022.
5. Yusuf AA, Matlon TJ, Grauer A, et al. Utilization of
osteoporosis medication after a fragility
fracture among elderly Medicare beneficiaries. Arch
Osteoporos. 2016;11:31.
This transcript is provided for your convenience and is qualified by the full video in which the spoken content appears. Please review the full video along with the transcript.
Fragility fractures at various sites are associated with significant clinical and personal burden.1
This may include: difficulty performing activities of daily living2,
admission to long-term care facilities,3
pain1 or complications from hospitalization,4
worry - which can impact relationships5, and
financial burden for patients6 and caregivers.7
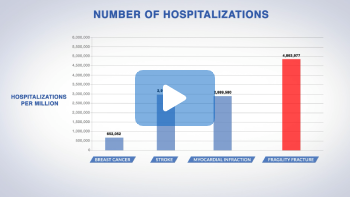
Fragility fractures result in more hospitalizations than breast cancer, stroke, or myocardial infarction.8
A prior fragility fracture can substantially increase the relative risk of a future fracture.9
And the subsequent fracture can occur at the same or a different site from the initial fracture.9
References:
1. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician's Guide
to Prevention and Treatment of
Osteoporosis. Osteoporos Int. 2014;25:2359-2381.
2. Fischer S, Kapinos KA, Mulcahy A, et al. Estimating the
long-term functional burden of osteoporosisrelated
fractures. Osteoporos Int. 2017;28:2843-2851.
3. Bentler SE, Liu L, Obrizan M, et al. The aftermath of hip
fracture: discharge placement, functional
status change, and mortality. Am J Epidemiol.
2009;170:1290-1299.
4. Inacio MC, Weiss JM, Miric A, et al. A community-based
hip fracture registry: population, methods,
and outcomes. Perm J. 2015;19:29-36.
5. Royal Osteoporosis Society. Life with Osteoporosis 2021:
the untold
story.
https://strwebprdmedia.blob.core.windows.net/media/1d5hdsg4/life-with-osteoporosis-
2021-public-report-final-1.pdf Accessed August 18, 2022.
6. Tarride JE, Hopkins RB, Leslie WD, et al. The burden of
illness of osteoporosis in Canada. Osteoporos
Int. 2012;23:2591-2600.
7. Kaffashian S, Raina P, Oremus M, et al. The burden of
osteoporotic fractures beyond acute care: the
Canadian Multicentre Osteoporosis Study (CaMos). Age
Ageing.
2011;40:602-607.
8. Singer A, Exuzides A, Spangler L, et al. Burden of
illness for osteoporotic fractures compared with
other serious diseases among postmenopausal women in the
United States. Mayo Clin Proc.
2015;90:53-62.
9. Gehlbach S, Saag KG, Adachi JD, et al. Previous fractures
at multiple sites increase the risk for
subsequent fractures: the Global Longitudinal Study of
Osteoporosis in Women. J Bone Miner Res.
2012;27:645-653.
10. Center JR, Bliuc D, Nguyen TV, et al. Risk of subsequent
fracture after low-trauma fracture in men
and women. JAMA. 2007;297:387-394.
This transcript is provided for your convenience and is qualified by the full video in which the spoken content appears. Please review the full video along with the transcript.
Obtain a DXA scan in all women ≥ 65 and women older than 50 who have clinical risk factors for osteoporosis.1
Bone mineral density alone does not explain all fragility fracture risk.
Understanding clinical risk factors and BMD together improve fracture risk prediction in these patients.1 Determining a patient’s fracture risk requires consideration of several clinical risk factors, of which a history of prior fracture, older age, and low bone mineral density are most important, followed by other non-modifiable and modifiable risk factors.1
There are several methods you can use to identify women over age 50 at high risk for fracture that need treatment.1 Patients with a history of fracture at the hip or spine are at a high risk for future fracture.3
Women over age 50 with bone mineral density T-scores below –2.5 are considered osteoporotic and at high risk for future fracture.1
High-risk patients are those women with FRAX 10-year probability of hip fracture ≥ 3%, or 10-year probability of major osteoporotic fracture ≥ 20%.3
Fragility fractures at the proximal humerus, pelvis, and in some cases, wrist qualify patients as high risk for future fracture, when occurring in combination with low bone mineral density at the hip or spine.1,4 Please note that regional thresholds and criteria for treatment eligibility may vary.1
References:
1. Camacho PM, Petak SM, Binkley N, et al. American
Association
Of Clinical
Endocrinologists/American College Of Endocrinology
Clinical
Practice Guidelines For The
Diagnosis And Treatment Of Postmenopausal
Osteoporosis-2020
Update. Endocr Pract.
2020;26(suppl 1):1-46.
2. Siris ES, Chen YT, Abbott TA, et al. Bone mineral
density
thresholds for pharmacological
intervention to prevent fractures. Arch Intern Med.
2004;164:1108-1112.
3. Shoback D, Rosen CJ, Black DM, et al. Pharmacological
Management of Osteoporosis in
Postmenopausal Women: An Endocrine Society Guideline
Update. J
Clin Endocrinol Metab.
2020;105:dgaa048.
4. Siris ES, Adler R, Bilezikian J, et al. The clinical
diagnosis of osteoporosis: a position statement
from the National Bone Health Alliance Working Group. Osteoporos
Int. 2014;25:1439-1443.
This transcript is provided for your convenience and is qualified by the full video in which the spoken content appears. Please review the full video along with the transcript.
Doctor: It’s not just a wrist fracture … your bone mineral density test at your hip indicated that you have osteoporosis because your T-score is below minus 2.5.1 Having this first fracture increases your risk for having another fracture in the future which could be at a different site.2 All of your other tests were normal. I recommend you start treatment for your osteoporosis.
Patient thought bubble 1 (read in a whispering voice by patient): Osteoporosis? I don’t know, that fall was an accident. I just need to focus on healing my wrist and then I’ll be more careful.3 Patient comment bubble 1 (read in a regular voice by patient): I have seen media reports about the side effects of those treatments. I need to do more research … I don’t want to start treatment just yet.1
Understand, acknowledge, and discuss your patient's concerns, but also ensure they understand that osteoporosis is a real disease that weakens their bones and makes them more likely to break.1,4
While the fears of treatment side effects are real, ensure your patient understands that osteoporosis is a chronic disease, and for many, the risks of the disease outweigh the risks of treatment.5
When discussing treatment options and the potential for adverse events, consider presenting statistics in an understandable way by using absolute numbers and visual aids.1
Encourage your patient to ask questions which promotes shared decision making and helps to identify what's most important to the patient and barriers to management.1
References:
1. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists/ American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 Update. Endocr Pract. 2020;26:1-46.This transcript is provided for your convenience and is qualified by the full video in which the spoken content appears. Please review the full video along with the transcript.
Osteoporosis is a very common bone disease, particularly among female patients after menopause.1
Today I would like to review with you the physiology, consequences of, and some tips on osteoporosis management.
I will also include updates to the clinical practice guidelines that come from the American Association of Clinical Endocrinologists (AACE).2
My name is Amanda McKee. I am a family nurse practitioner. I'm also an orthopedic nurse practitioner and a certified clinical densitometrist.
This disease awareness program is presented on Amgen's behalf and has been reviewed with consistent with Amgen's internal review policy.
Speaker disclosures…
Let’s first set the stage so that we have a common lexicon.
The World Health Organization has defined osteoporosis as a disease characterized by low bone mass and structural deterioration of bone tissue, and these two conditions lead to bone fragility as well as an increased risk of fractures.3
And a fracture is the clinical end result that we want to prevent, because as you may be well aware of, a fracture can be debilitating to patients.4
Before we can discuss the pathophysiology of osteoporosis, we need some of the basics of bone anatomy and physiology.
At the center here is an illustration of typical long bone – such as a femur in the thigh, or the radius in the forearm. There are 2 major components – the trabecular bone (which is the inside portion) and the cortical bone (which is on the outside).5
The trabecular bone is a honeycomb-like network of delicate, interconnected plates that looks like a sponge.5,6
Although it seems to occupy a lot of space, it represents only 20% of total bone mass.6
The trabecular bone acts as major reserve for calcium and phosphate in the body, and provides a large surface area for mineral exchange.7,1
The orientation of the trabecular plates also allows for maximal strength without much of the bulk, similar to how we design buildings and bridges.1
Finally, the trabecular bone is the major site for bone remodeling, which we will discuss in more detail later, and has a higher turnover rate.2,7
Next, the cortical bone is quite different. It is dense, it defines the shape of the bone, and it accounts for most of the skeletal mass.1
Functionally, the cortical bone provides biomechanical strength, it serves as attachment sites for tendons and muscles that are associated with movement, and it protects organs that the bone surrounds such as the bone marrow and the brain. 1,7,8
The turnover rate for cortical bone is slow, at 2%–3% per year, which is sufficient for maintaining the biomechanical strength of the bone.8
What is bone remodeling, and why do we need it? Bone remodeling is a normal, physiological, and continuous process that is needed to maintain a healthy skeleton.1
Remodeling repairs damage to the skeleton that can result from stresses. It
prevents accumulation of old, brittle bone.
And it is important for the function of the skeleton as the storage for calcium and phosphorous. In
fact, most of the adult skeleton is replaced about every 10 years.1
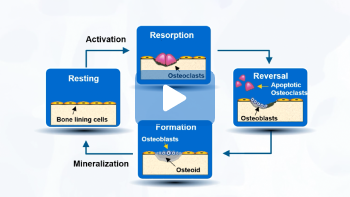
Bone remodeling follows a specific sequence of events. Let’s start by orienting ourselves to the Resting stage of the cycle. Here, the lining cells occupy the surface of the bone.9
[Click] It has been suggested that certain bone cells can sense bone stress that is caused by mechanical loading or microdamage to the bone, and activates the remodeling cycle. 7,10
During the Resorption phase, cells called osteoclasts are recruited to the bone. These cells secrete various enzymes and acids to destroy the old bone matrix.9,10
[Click] The next phase is called Reversal. During this phase, a new group of cells called osteoblasts to the site where the osteoclasts have vacated.10
[Click] Next is the Formation phase. Now, the osteoblasts are responsible to lay down new bone matrix –
this organic component is called osteoid – to fill up the hole.7,9
As a final step, the osteoid undergoes a process called mineralization, and completes the remodeling process for the bone.10
The entire process itself takes approximately 3–6 months.9
In normal bone remodeling, bone formation and bone resorption is balanced, so there are no major net changes in bone mass or mechanical strength after each cycle.7
However, In osteoporosis, the bone remodeling process is out of balance because bone resorption exceeds bone formation. There can be a variety of reasons that can lead to this remodeling the slowdown in bone formation, or an acceleration of bone resorption, or both.1
One important cause of osteoporosis is a decrease in the production of sex hormones, both estrogen and testosterone, which leads to an increase in osteoclast activity, an acceleration in bone resorption, a lower bone mineral density, and eventually increasing the risk of fracture.1,11
In postmenopausal women, there is an increase in bone resorption that’s primarily associated with a deficiency in estrogen. Postmenopausal women are therefore at high risk for developing osteoporosis.11-13
Let’s go just one level deeper to explain the relationship between a decline in estrogen level and excessive bone remodeling. There are many participants in the bone remodeling process.
[Click] But for the purpose of this discussion, we are going to focus on the relationship of these five players.
[Click] First, the discovery of the essential role of RANK ligand pathway which was a major milestone in the understanding of bone regulation and bone pathology, including postmenopausal osteoporosis.7
RANK ligand is a cytokine that can be bind that can bind to its receptors on osteoclasts. This increases the number and activity of osteoclasts. Therefore, more RANK ligand leads to more bone resorption.7,11
[Click] It turns out that there is another receptor for RANK ligand, called OPG. OPG is a soluble decoy receptor that is derived from osteoblasts, and can bind to RANK ligand, and as a result antagonizing bone resorption. 7,10,11,14
[Click] Estrogen exerts its bone-sparing effects by targeting this system, but indirectly. It turns out that estrogen has a profound impact on osteoblasts, the bone-forming cells. Estrogen signals the osteoblasts to increase production of OPG, resulting in a reduction in RANK ligand and bone resorption.7,11
In postmenopausal women, the lack of estrogen leads to an increase in RANK ligand, and its ability to stimulate bone resorption, increasing the risk of this population in developing osteoporosis and fracture.7
Let’s take a closer look at the change in bone mass throughout the life span. Bone accumulates rapidly in childhood and grows rapidly during puberty. Peak bone mass is reached by age 30, and then slowly decreases as we get older. This is observed in both males and females.15
There are a couple of notable differences between genders. In general, bone mass in men is higher than women. After 40 years old, bone mass in men gradually declines.15
In contrast, females typically accumulate less bone mass than males, and rapidly lose bone during menopause.15
And according to National Osteoporosis Foundation, as much as 20% of their bone mass can be lost during the first 5 to 7 years following the start of menopause.16
So, many women in their 50’s may already have low bone mass, making them more susceptible to osteoporosis later in life.15
The image in the top panel shows what normal bone looks like.
As we age, bone formation tends to decrease, usually failing to keep up with the rate of bone resorption. The imbalance between bone resorption and bone formation leads to a loss in bone mass, and the development of structural abnormalities.1
This result is how we are characterizing osteoporosis:
Disruption to bone architecture17
Compromised bone strength17
And an increased risk of fracture.4
Now that we have a high-level overview of bone physiology and osteoporosis pathology, let’s turn our attention to the burden of osteoporosis.
One study estimated the prevalence of osteoporotic fractures to be over 2 million based on data from 2005. And more alarming is that the projection for the year 2025 –is only 5 years away – is expected to be about 3 million. This represents a 48% increase in osteoporotic fractures in just over 20 years.13
Furthermore, approximately 50% of women 50 years and older in the United States are expected to experience an osteoporotic fracture some time during their remaining years.18
While breast cancer garners significant attention when we talk about diseases in women in the United States, you may be surprised to know that the incidence of osteoporosis-related fractures is actually a lot higher than breast cancer diagnosis.19
In 2006 among women of all ages – not just the ones over 50 years old –number of new cases of breast cancer was just over 200,000 a year.19
In contrast, in 2005 just hip fractures alone accounted for 200,000 cases, not to mention the fractures of the spine, the wrist, and other sites. Overall, the annual incidence of osteoporotic-related fracture among women of all ages was 1.4 million, or 6-fold higher than breast cancer diagnosis.19
Osteoporosis is a "silent" disease, meaning that the first sign of the disease is often a fracture.4
Fractures occurring at certain key sites in women over the age of 50 should be a signal to healthcare professionals to evaluate osteoporosis further.4,20
Major sites of osteoporosis-related fractures are highlighted here, with vertebral fractures being the most common, followed by fractures of the wrist, and then fractures of the hip.4,18,19
Of note, the majority of vertebral fractures are initially relatively asymptomatic, so it is not surprising that it remains undetected in approximately two-thirds of the cases.3,21
Although wrist fractures are typically less debilitating, but it can interfere with some activities of daily living.4
It should not be surprising that a hip fracture cause the most debilitating osteoporosis complications there is.4,22
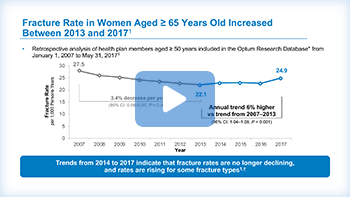
The establishment of screening and treatment guidelines for osteoporosis have facilitated a decline in fracture rates between 2007 and 2013.23
Specifically among women 65 years and older, the fracture rate dropped from 27.5 fractures for every 1,000 person-years, down to about 22. This represents a significant decrease of 3.4% per year.23
However, in more recent years, the fracture rate has reversed course and has been climbing up again.23
The data points shown in blue on this graph show a relative plateau since 2013, then an increase in fracture rates among women over the age of 65 in 2017.23
Many factors may have played a role in contributing to the increasing fracture rates.23,24
Since 2007, Medicare reimbursement for bone density screening was cut far below the actual cost of performing a DXA – or dual-energy x-ray absorptiometry – scan. As a result, these services were less likely be offered by rheumatologists, physicians in solo practices, and practices where access to fewer than three DXA scanners.24
Osteoporosis-related fractures can have a negative impact on the patient. Let’s take a look at some of the major issues.
An osteoporosis-related fracture may prevent a person from caring for herself; for this reason, she may require admission to a nursing home or a long-term care facility.25
Next, Costs due to osteoporosis-related fracture can create a heavy financial burden for the affected person and their caregivers.26,29
The patient may not be able to do some of the regular activities of daily living because of the fracture.4,25,30
Then they may Worry about falls, the possibility of future fractures, and the potential need for nursing home care is sometimes experienced by a person with an osteoporosis-related fracture.27,31
And finally, complications such as chronic pain and other complications may occur as well. 4,32
Once a patient experiences a fracture, the risk of having a subsequent fracture goes up.33
A study published in JAMA followed a cohort of ambulatory patients over a 16-year period. These patients lived in community and not in a nursing home.33
Among elderly women who had a prior fracture, researchers found the risk of an initial fracture went up with age.33 In addition, the risk for a subsequent fracture went up by 1.6 to 2.4 times.33
In fact, the risk of a subsequent fracture amongst women aged 60–69 years old who had a prior fracture (36%), was higher than the risk of an initial fracture amongst women aged 70–79 years old (at 27%).33
The same goes with those in the age of 70–79 who had a prior fracture. Their risk for a subsequent fracture (63%) was higher than the risk of an initial fracture in those who were 80 years of age or older (at 50%).33
This data underscores the fact that the increased risk of a subsequent fracture persists for up to 10 years depending on age and sex.33
I always like to say that a fracture begets a fracture.20
In fact, when we examine the period following an osteoporosis-related fracture more closely, it becomes more clear that the highest risk of a re-fracture is within the first year after the initial fracture.34
This can be illustrated using data from a population-based study over 4,100 postmenopausal women.34
In this study, fractures included hip fractures, major fractures (which include clinical vertebral fractures, forearm, and humerus), and minor fractures (all others), as classified by the World Health Organization.34
The relative risk of a subsequent fracture is 5 times greater in the first year after a fracture compared with the risk of a first fracture. In fact, one of every four subsequent fractures happen within the first year after the initial fracture. This supports early action in prevention of a subsequent fracture.34
Again, remember that a fracture begets a fracture.20
Although various types of fractures can occur in postmenopausal women with osteoporosis, vertebral fractures are independent risk for future fractures.4,35One of every four women who experience a vertebral fracture will have a second fracture within 2 years.35
In fact, the risk of the second fracture being another vertebral fracture is 7.3 times higher among women aged 55 years or older with a history of a vertebral fracture.36
However, most vertebral fractures go undiagnosed at the time that they occur due to lack of symptoms. 21,37,38
Because of increased risk of second fracture, it is most important to identify those postmenopausal women who have a vertebral fracture.39 Candidates for assessment may have one or more of the following characteristics:
Significant height reduction, either historically with a difference of 1.5 inches or more between the peak height at age 20, or prospectively with a difference of 0.8 inches or more between a previously documented height measurement compared to the current height4,39
They may also have Postural changes including stooping or progressive spinal curvature1,39 Or worsening or unexplained back pain39
To detect vertebral fractures, vertebral fracture assessments – also known as VFA – imaging, or lateral x- ray are often used.37,39
Among all the osteoporotic fractures, the worst consequences can occur with hip fractures.22
It is not surprising then like those with vertebral fractures are increased risk of a subsequent vertebral fracture, those with hip fractures are at increased risk of future hip fractures.36
In fact, women who have hip fracture have a 3.5-fold greater risk of a second hip fracture.36
[Click] In addition, the probability of needing care in long-term nursing facility is increased 4-fold after a hip fracture.26
[Click] Despite these increased risks, a study found that only ~11% to 13% of patients receive pharmacological therapy for osteoporosis within 3 months of the hip fracture.22
How good does BMD by itself predict the risk of fracture?I would like to share with you results of a large observational study done in the United States, which involved approximately 150,000 women who were white, postmenopausal, over 50 years of age, and were NOT diagnosed with osteoporosis. A baseline BMD T-score was measured, and new fractures over the following 12 months were documented.40
The x-axis is the BMD T-score, with the best score to the left and the worst to the right.
The green dotted curve shows distribution of BMD T-scores, which approximates a normal distribution.2,40
The blue columns are the fracture rates. There was a strong continuous relationship between lower BMD T-scores and a higher fracture rate, which could not be too surprising. Notice also that fractures did occur in those women who did not have a BMD T-score in the osteoporotic range.40
It is crucial to recognize that these fractures are associated with skeletal fragility even though they do not have a T-score in osteoporosis range.40
[Click] The real insight comes when we consider these two findings together. The red bars here represent the absolute number of women with new fractures based on BMD T-scores.40
A large number of osteoporotic-related fractures occurred in patients with low bone mass, because of the large number of patients with bone mass in this range.4
Take a look at the red bars inside the gray box. These were women who experienced a new fracture, despite having a BMD T-score of better than a –2.5. And this represents over 80% of women with a new fracture.40
What this study shows is BMD T-scores alone is not a very good predictor of future risk of having a fracture.40
If a low bone density alone is not a very good predictor of future risk of fracture, then what else is there?
It turns out there are a whole host of risk factors that have been associated with an increased risk of osteoporosis-related fractures.4
These range from prior fracture, age, to parental history of hip fracture, low body mass index, immobilization, long-term glucocorticoid use, alcohol use, smoking, rheumatoid arthritis, diabetes, and whether there is a high risk of falling. This is just a abbreviated short list.2,4
In general, there are more risk factors that are present, the higher risk there is for a future of fracture.4
One of these risk factors – having a prior fracture – is particularly important predictor of future fracture risk.2
As we have discussed before, postmenopausal women who had an osteoporotic-related fracture were found to be 5 times more likely to suffer another fracture within the first year following.34
And in fact, as we will review later, prior fracture is being emphasized in the latest guideline in diagnosing osteoporosis.2
The first step in managing osteoporosis is to properly establish a diagnosis. It is important to note that the guidelines point out osteoporosis can be diagnosed with a low BMD, or a history of fracture, or some other combinations.2
This year the American Association of Clinical Endocrinologists (AACE) updated its guidelines for the diagnosis and treatment of postmenopausal osteoporosis.2
According to these guidelines, a diagnosis of osteoporosis can be made if ANY ONE of the following 4 criteria are met:2
The first is having a BMD T-score of –2.5 or worse in any of the major sites. This is a group of patients we traditionally think of when we think about osteoporosis. However, diagnosis can also be made with the following criteria as well:
A low-trauma fracture of the hip, the spine, regardless of BMD score, OR
A T-score T-score between –1 and –2.5, PLUS a fragility fracture of proximal humerus, pelvis, or distal forearm, OR
a T-score of –1 to –2.5, PLUS a high FRAX® fracture probability. If a trabecular bone score-adjusted FRAX® score is available, use that instead.
Once a diagnosis of osteoporosis is made, the diagnosis persists for the rest of the patient’s life. And this is true even if aggressive management results in an improvement of the BMD T-score to be better than – 2.5.2
There are a number of reasons for this:
First, we know that osteoporosis is a chronic, progressive silent disease, much like hypertension or diabetes.41
The goal of management is to prevent fractures, but no treatment can eliminate the risk of fracture.2
There is also no consensus of what an acceptable level of fracture risk should be, whether based on BMD measurement or monitoring of FRAX® scores.2
Therefore, management of osteoporosis needs to be long-term, once a diagnosis is made.2
For example, AACE guidelines endorse lifelong physical activities for osteoporosis prevention.2
So how do we identify patients who may be at high risk of osteoporosis fractures?
First, If a patient is 50 or older, and is postmenopausal, evaluate for risk of osteoporosis.2
We can look for signs or symptoms to see if further testing is needed. These further tests may include BMD measurements, or vertebral imaging to detect future fracture.2
For example, does the patient have unexplained back pain? 2
Or Does the patient have kyphosis – which is an abnormal spinal curvature?2
How about an unexplained loss of height? Ideally, this is measured once a year using a wall-mounted stadiometer.2,4
This loss of height can be defined two different ways. A historical height loss difference between the patient’s current height versus peak height age 20. A loss of 1.5 inches, or 4 cm, is associated with a new vertebral fracture.2,4
Another definition is called prospective height loss, which is the height which is the difference between the patient’s current height and a previously recorded height. A prospective height loss of 0.8 inches or more than 2 cm is also associated with a new vertebral fracture.2,4
[Click] You should ask the patient questions related to possible osteoporosis risk factors.4
For example, is there a parental history of hip fracture?
Is the patient using any medication that may be associated with bone loss?
Once a patient has been found to be at high risk for fracture and osteoporosis, a central DXA scan is usually ordered to measure the bone density, usually of the hip and the spine.2,42
Only results from a central DXA can be used to establish a diagnosis of osteoporosis, because peripheral tests such as the peripheral dual energy x-ray absorptiometry (pDXA), quantitative ultrasound (QUS), and peripheral quantitative computed tomography (pQCT) are useful for screening. Moreover, results of a peripheral DXA cannot be compared to that of a central test.42
DXA usually takes about 10 minutes to perform.16,42 And
it is used to obtain a patient’s BMD T-score.42
In addition to its role in diagnosis, DXA is also used to monitor treatment for osteoporosis.2
The AACE 2020 guidelines recommend an axial DXA scan every 1 to 2 years until the findings are stable.2,42
Medicare Part B covers DXA scan once every 24 months to monitor osteoporosis treatment if certain conditions are met.43
Ideally, repeat DXA scans should be done at the same location, using the same equipment for the most accurate comparison of results.42
As I mentioned earlier, the T-score obtained with a DXA scan can be used to determine fracture risk and diagnose osteoporosis.2
The T-score is defined as standard deviation of an individual’s bone mineral density (BMD) from the mean value for young normal white women.2
As the T-score decreases, the BMD decreases. And as BMD decreases, the risk of fracture increases.2
This slide shows a range of T-scores and their relationship to bone health.
The left end of this illustration shows T-scores ranging from −1.0 and 0, which indicate normal bone density. At the right end of the figure are T-scores of −2.5 or lower; as I pointed out earlier, a T-score that is less than or equal to −2.5 is sufficient for diagnosis of osteoporosis. Between the T-scores for normal bone and osteoporotic bone are the T-scores indicating osteopenia. Keep in mind that osteoporosis can be diagnosed if the T-score is between −2.5 and −1.0 and the patient has had a prior fragility fracture of the proximal humerus, pelvis and/or distal forearm.2,4
It is possible that a T-score may increase and surpass −2.5 with therapy; regardless, osteoporosis persists despite this improvement.2
A patient’s 10-year risk of fracture can be assessed with tools such as a FRAX®, which is an algorithm designed by the World Health Organization for use in primary care.44
The risk is calculated based on clinical factors can be easily collected, as summarized on the left side of the slide.44
Data regarding the risk factors can be entered directly into the FRAX® calculator online, as depicted in the middle portion of this slide.
Once the clinical data has been entered, the FRAX® calculator will generate a 10-year probability of a hip fracture and a 10-year probability of a major osteoporosis-related fracture.44
The right side of this slide shows how the output should be analyzed, based on the thresholds at which osteoporosis treatment is expected to be cost-effective.45
The intervention threshold for hip fracture risk is 3% or greater. 45
Likewise, the intervention threshold for a major osteoporotic fracture is 20% or greater.45
Note that we refer to a “major” osteoporotic fracture as a fracture of the hip, the spine, the humerus, or the wrist.44
Although primary care providers often diagnose and manage osteoporosis, a referral to endocrinologist or other osteoporosis specialist may be considered if the patient has one or more of the following characteristics shown on this slide:2
A normal BMD and a fracture due to a nontraumatic event. A normal BMD is defined as a T-score −1.0 or above, however, patients with low bone mass or osteopenia with a T-score between −1.0 and −2.5 with a fragility fracture are also increased risk for future fracture events.
A recurrent fracture or ongoing bone loss despite therapy for osteoporosis and no other apparent cause of bone loss. Also, Secondary conditions, such as hyperthyroidism, hyperparathyroidism, hypercalciuria, or increased prolactin.
Conditions that may complicate treatment, such as renal impairment, malabsorption, or hyperparathyroidism.
Also, Severe osteoporosis or osteoporosis with unusual features, such as young age, a low phosphorus, abnormal alkaline phosphatase, or abnormal lab results.
Also, Unexplainable artifacts on DXA scans. Or
Osteoporosis-related fracture.
An osteoporosis-related fracture is one that occurs in a patient with osteoporosis who experiences minimal trauma, such as a fall from standing height or less.1
The risk of fracture stems not only from bones becoming more fragile, but also from the increased risk of falls due to loss of muscle strength and function with age. After the age of 30, approximately 5% of muscle mass is lost with every decade of life, and this rate only accelerates beyond age 65. It is not surprising then that one in every four women age 50 or older and one in two women age 85 or older, falling annually.1
Falls contribute to 25% of the clinically diagnosed vertebral fractures, whereas the majority of clinically diagnosed fractures result from excess stresses on the spine caused just by everyday activities.1
If an adult older than 50 years has a fracture at any major skeletal site, consider that a warning sign that this patient may have low bone density or osteoporosis. Further evaluation of the patient is required.4
Today, osteoporosis is managed by lifestyle modifications as well as pharmaceutical therapy.16,46
Lifestyle modifications include getting sufficient calcium and vitamin D, engaging in exercise to improve strength and balance, avoiding tobacco, limiting alcohol use, and taking steps to prevent falls, which can cause fracture. 2,16,47
Calcium supplements of 1,200 mg/day for women age 50 and older and vitamin D is recommended.2
Weight-bearing exercises such as walking for 30–40 minutes and posture exercises for a few minutes should be done regularly (which is 3–4 days per week) muscle strengthening exercises such as lifting weights are important for building and maintaining bone density. 2,48
Also, cigarette smoking is known to be detrimental to bone health, although it is not clear whether it is due to enhanced metabolism of estrogen or direct effects on bone metabolism.2
Since excessive alcohol intake is also associated with increased risk of fracture, for postmenopausal women at high risk for osteoporosis consumption of alcohol should be limited to 2 drinks daily, with each drink being equivalent to 120 mL of wine, or 30 mL of liquor, or 260 mL of beer.2
Fall prevention strategies include optimizing drugs that affect the central nervous system or blood pressure, minimizing environmental factors such as poor lighting or loose rugs, and improving strength and balance.1,2
There are also a variety of pharmaceutical therapies that are target the different pathophysiological pathways of osteoporosis.2
In this Expert Theater, we have discussed three areas about postmenopausal osteoporosis and osteoporotic-related fractures.
The reduction estrogen after menopause upsets the balance in bone remodeling through its effect on the RANK ligand pathway, resulting in excessive bone resorption and increased risk of fracture.7
Clinically, a fracture can result in significant disability and loss of independence for the patient.4
In addition, once a patient has a fracture, she is at a much higher risk of having another one, especially in the next 1–2 years following.34
Therefore, it is important for us to identify patients who are at risk for osteoporosis as early as possible.
For every patient over the age of 50, we can routinely evaluate by looking for unexplained back pain or height loss, and ask about other risk factors.2,4
If you know that the patient recently has a fracture at any of the major skeletal sites, it is a trigger for us to investigate further.4
We also have tools – such as a DXA scan, vertebral imaging, and FRAX® risk assessment – that can further guide our actions with those patients we consider to be at high-risk, whether to establish a diagnosis, or refer to a specialist. 2,4,42,44
Finally, we need to manage osteoporosis like a chronic,
progressive disease with a long-term goal of
reducing the risk of fractures risk. Once a diagnosis of
osteoporosis is made, the patient will need to be
monitored and treated for the long haul.2,4
I hope this discussion has provided you with some
insight
and practical guidance into the epidemiology
and physiology of osteoporosis and osteoporotic
fracture in
women after menopause, as well as some
practical guidance in the diagnosis and management. If you have any question whatsoever, please contact
Amgen
Medical Information. And I would like to thank you for joining me throughout
this
presentation. References:
1. US Department of Health and Human Services. Bone
health
and osteoporosis: a report of the
surgeon general. 2004.
2. Camacho PM, Petak SM, Binkley N, et al. American
Association of Clinical
Endocrinologists/American College of Endocrinology
clinical
practice guidelines for the diagnosis
and treatment of postmenopausal osteoporosis-2020
update.
Endocr Pract. 2020;26(suppl1):1-
46.
3. WHO Study Group. Assessment of fracture risk and
its
application to screening for
postmenopausal osteoporosis. Report of a WHO Study
Group.
World Health Organ Tech Rep
Ser. 1994;843:1-129.
4. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician's
guide
to prevention and treatment of
osteoporosis. Osteoporos Int. 2014;25:2359-2381.
5. Willems NMBK, Langenbach GEJ, Everts V, Zentner
A.
The
microstructural and biomechanical
development of the condylar bone: a review. Eur J
Orthod.
2014;36:479-485.
6. Dempster DW. Primer on the Metabolic Bone
Diseases
and
Disorders of Mineral Metabolism.
6th ed. 2006:7-11.
7. Feng X, McDonald JM. Disorders of bone
remodeling.
Annu
Rev Pathol Mech Dis. 2011;6:121-
145.
8. Clarke B. Normal bone anatomy and physiology.
Clin J
Am
Soc Nephrol. 2008;3(suppl 3):S131-
S139.
9. Baron R. General principles of bone biology. In:
Primer
on the Metabolic Bone Diseases and
Disorders of Mineral Metabolism. 5th ed. 2003:1-8.
10. Baron R, Hesse E. Update on bone anabolics in
osteoporosis treatment: rationale, current status,
and perspectives. J Clin Endocrinol Metab.
2012;97:311-325.
11. Michael H, Harkonen PL, Vaananen HK, Hentunen
TA.
Estrogen and testosterone use different
cellular pathways to inhibit osteoclastogenesis and
bone
resorption. J Bone Miner Res.
2005;20:2224-2232.
12. Raisz LG. Pathogenesis of osteoporosis:
concepts,
conflicts, and prospects. J Clin Invest.
2005;115:3318-3325.
13. National Osteoporosis Foundation. What women
need to
know. Available at:
www.nof.org/preventing-fractures/general-facts/what-women-need-to-know/.
Accessed
October 5, 2020 .
14. Boyle WJ, Simonet WS, Lacey DL. Osteoclast
differentiation and activation. Nature.
2003;423:337-342.
15. Hodges JK, Cao S, Cladis DP, Weaver CM. Lactose
intolerance and bone health: the challenge of
ensuring adequate calcium intake. Nutrients.
2019;11:718.
16. National Osteoporosis Foundation. General facts.
Available at: www.nof.org/preventingfractures/
general-facts/. Accessed September 21, 2020.
17. NIH Consensus Development Panel on Osteoporosis
Prevention, Diagnosis, and Therapy.
Osteoporosis prevention, diagnosis, and therapy.
JAMA.
2001;285:785-795.
18. Burge R, Dawson-Hughes B, Solomon DH, Wong JB,
King
A, Tosteson A. Incidence and economic
burden of osteoporosis-related fractures in the
United
States, 2005-2025. J Bone Miner Res.
2007;22:465-475.
19. Watts N, Bilezikian JP, Camacho PM, et al.
American
Association of Clinical Endocrinologists
medical guidelines for clinical practice for the
diagnosis and treatment of postmenopausal
osteoporosis. Endocr Pract. 2010;16(suppl 3):1-37.
20. International Osteoporosis Foundation. Capture
the
fracture. Available at:
www.capturethefracture.org/about. Accessed September
21,
2020.
21. Cooper C, Atkinson EJ, O’Fallon WM, Melton LJ
3rd .
Incidence of clinically diagnosed vertebral
fractures: a population-based study in Rochester,
Minnesota, 1985-1989. J Bone Miner Res.
1992;7:221-227.
22. Kim SC, Kim MS, Sanfelix-Cimeno G, et al. Use of
osteoporosis medications after hospitalization
for hip fracture: a cross-national study. Am J Med.
2015:128:519-526.
23. Lewiecki EM, Chastek B, Sundquist K, et al.
Osteoporotic fracture trends in a population of US
managed care enrollees from 2007 to 2017. Osteoporos
Int. 2020;31:1299-1304.
24. Hayes BL, Curtis JR, Laster A, et al.
Osteoporosis
care in the United States after declines in
reimbursements for DXA. J Clin Densitom.
2010;13:352-360.
25. Bentler SE, Liu L, Obrizan M, et al. The
aftermath
of hip fracture: discharge placement,
functional status change, and mortality. Am J
Epidemiol.
2009;170:1290-1299.
26. Tajeu GS, Delzell E, Smith W, et al. Death,
debility, and destitution following hip fracture. J
Gerontol A Biol Sci Med Sci. 2014;69:346-353.
27. National Osteoporosis Society. Life with
Osteoporosis. October 2014. Available at:
https://theros.org.uk/media/1859/life-with-osteoporosis.pdf.
Accessed September 21, 2020.
28. Tarride J-E, Hopkins RB, Leslie WD, et al. The
burden of illness of osteoporosis in Canada.
Osteoporos Int. 2012;23:2591-2600.
29. Kaffashian S, Raina P, Oremus M, et al. The
burden
of osteoporotic fractures beyond acute care:
the Canadian Multicentre Osteoporosis Study (CaMos).
Age
Ageing. 2011;40:602-607.
30. Fischer S, Kapinos KA, Mulcahy A, Pinto L,
Hayden O,
Barron R. Estimating the long-term
functional burden of osteoporosis-related fractures.
Osteoporos Int. 2017;28:2843-2851.
31. Vass CD, Sahota O, Angelova T. Fear of falling
after
fragility fracture–a prevalence study. Age
Ageing. 2014;43:i29.
32. Inacio MCS, Weiss JM, Miric A, Hunt JJ, Zohman
GL,
Paxton EW. A community-based hip
fracture registry: population, methods, and
outcomes.
Perm J. 2015;19:29-36.
33. Center JR, Bliuc D, Nguyen TV, Eisman JA. Risk
of
subsequent fracture after low-trauma fracture
in men and women. JAMA. 2007;297:387-394.
34. van Geel TACM, van Helden S, Geusens PP, Winkens
B,
Dinant G-J. Clinical subsequent fractures
cluster in time after first fractures. Ann Rheum
Dis.
2009;68:99-102.
35. Roux C, Fechtenbaum J, Kolta S, Briot K, Girard
M.
Mild prevalent and incident vertebral
fractures are risk factors for new fractures.
Osteoporos
Int. 2007;18:1617-1624.
36. Gehlbach S, Saag KG, Adachi JD, et al. Previous
fractures at multiple sites increase the risk for
subsequent fractures: the Global Longitudinal Study
of
Osteoporosis in Women. J Bone Miner
Res. 2012;27:645-653.
37. Gehlbach SH, Bigelow C, Heimisdottir M, May S,
Walker M, Kirkwood JR. Recognition of
vertebral fracture in a clinical setting. Osteoporos
Int. 2000;11;577-582.
38. Nevitt MC, Ettinger B, Black DM, et al. The
association of radiographically detected vertebral
fractures with back pain and function: a prospective
study. Ann Intern Med. 1998;128:793-800.
39. The International Society for Clinical
Densitometry.
VFA patient information. Available at:
www.iscd.org/patient-information/vfa/. Accessed
September 21, 2020.
40. Siris E, Cheng YT, Abbott TA, et al. Bone
mineral
density thresholds for pharmacological
intervention to prevent fractures. Arch Intern Med.
2004;164:1108-1112.
41. National Osteoporosis Foundation. Clinician's
guide
to prevention and treatment of
osteoporosis. Washington, DC: National Osteoporosis
Foundation; 2014.
42. National Osteoporosis Foundation. Bone density.
Available at:
www.nof.org/patients/diagnosisinformation/
bone-density-examtesting/. Accessed September 21,
2020.
43. Medicare. Bone mass measurements. Available at:
www.medicare.gov/coverage/bone-massmeasurements.
Accessed September 21, 2020.
44. Kanis JA, Hans D, Cooper C, et al.
Interpretation
and use of FRAX in clinical practice. Osteoporos
Int. 2011;22:2395-2411.
45. Kanis JA, Johansson H, Oden A, Dawson-Hughes B,
Melton LJ 3rd, McCloskey EV. The effects of a
FRAX revision for the USA. Osteoporos Int.
2010;21:35-40.
46. National Institute of Health. Osteoporosis
overview.
Available at:
https://www.bones.nih.gov/health-info/bone/osteoporosis/overview.
Accessed October 5,
2020.
47. Eastell R, Rosen CJ, Black DM, Cheung AM, Murad
MH,
Shoback D. Pharmacological
management of osteoporosis in postmenopausal women:
an
Endocrine Society clinical practice
guideline. J Clin Endocrinol Metab.
2019;104:1595-1622.
48. National Osteoporosis Foundation. Osteoporosis
exercise for strong bones. Available at:
https://www.nof.org/patients/treatment/exercisesafe
movement/osteoporosis-exercise-forstrong-
bones/. Accessed November 1, 2020.
This transcript is provided for your convenience and is qualified by the full video in which the spoken content appears. Please review the full video along with the transcript.
The annual number of fractures in women at least 65 years old in the US is projected to increase in the next two decades.1
Diagnostic screening is critical for reducing the risk of fragility fractures.2
Bone Mineral Density, as measured by a DXA scan, is the clinical standard for diagnosing osteoporosis.3
Yet, while DXA testing is well established, fewer than 10% of women and 2% of men in the eligible Medicare population were tested each year.4,5
A Medicare-reimbursed bone test called Biomechanical Computed Tomography analysis, or BCT, is now available.2
Because it can utilize most CT scans taken for any medical indication that capture the hip or spine, BCT can provide a fracture risk assessment without the need for an extra patient procedure or additional radiation exposure.2
Let’s consider a hypothetical clinical case of a 70-year-old woman that presents to the ER with unexplained stomach pain and undergoes an exploratory pelvic-abdominal CT.
In reviewing her medical history, her physician incidentally notes that she is at increased risk of osteoporosis due to age,3 but has not received an osteoporosis test in recent years. So he orders a BCT test and the patient’s pelvic abdominal CT scan is sent from the hospital to a BCT testing facility.
There, a biomechanically-accurate 3D model of the patient’s hip or spine is created from the scan data. 2
The model is then subjected to a virtual stress test that simulates a sideways fall for the hip or a compressive overload for the spine, with the regions of tissue failure colored red thus assessing the bone’s breaking strength and fracture risk.2
The results of the BCT test are summarized in a report that can include hip BMD T-scores suitable for use with FRAX®. 2
Based on both the BMD and bone strength measurements BCT can be used to diagnose osteoporosis and provides an overall fracture risk classification of: high risk, increased risk, or not-increased risk.2
The BCT medical report is then returned to the ordering physician who uses it to assemble a patient care plan.
As a newer clinical test, BCT is not yet widely adopted. And while BCT can be applied to most clinical CT scans that contain the proximal femur or lower spine, analysis may not be possible due to factors such as poor image quality or the presence of metal implants.2
In summary, the BCT clinical test can provide mechanistic insight about bone strength and fracture risk while helping to address the osteoporosis diagnosis gap.2,6
References:
1. Lewiecki EM, et al. JBMR Plus. 2019;3:e10192.
2. Keaveny TM, et al. Osteoporos Int.
2020;31:1025-1048.
3. Camacho PM, et al. Endocr Pract. 2020;26(suppl
1):1-46
4. Lewiecki EM, et al. J Clin Densitom.
2016;19:127-140
5. Zhang J, et al. J Bone Miner Res. 2012;27:858-864
6. Adams AL, et al. J Bone Miner Res.
2018;33:1291-1301.
This transcript is provided for your convenience and is qualified by the full video in which the spoken content appears. Please review the full video along with the transcript.
Welcome to this video on a review of recently updated postmenopausal osteoporosis guidelines.
I am Dr. Steven Petak.
This disease awareness program is presented on Amgen’s behalf, and has been reviewed consistent with Amgen’s internal review policies
Here are my disclosures
I am also member of the Amgen and Alexion speaker bureaus.
Over the past two years, several societies have released updated Clinical Practice Guidelines for postmenopausal osteoporosis. This includes the American Association of Clinical Endocrinologists and American College of Endocrinology, the International Osteoporosis Foundation and European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis, and the Endocrine Society.1-3
These updated guidelines include recommendations intended to optimize patient care that are informed by a systematic review of evidence and an assessment of the benefits and harms of alternative care options.1-4
There are some common themes between the updated postmenopausal osteoporosis guidelines as they focus on fracture risk assessment and treatment strategies.1-3
Fracture risk stratification is emphasized, and categorizes patients into high risk, and a new, very-highrisk risk category. ENDO and IOF-ESCEO Guidelines include both a low risk category, and ENDO additionally includes a moderate risk category.1-3
Treatment is recommended in patients at high and very high risk, and is individualized based on a patient’s risk classification.1-3
Given the chronic nature of osteoporosis, long-term treatment strategies should be considered, including sequencing. Anabolic agents are recommended as initial therapy for patients at very high risk, and AACE guidelines also include injectable anti-resorptives as an initial therapy option in very high risk patients. All three guidelines state that anabolic therapy should be followed with subsequent antiresorptive therapy.1-3
AACE, ENDO, and IOF-ESCEO guidelines all contain recommendations for fracture risk assessment.1,2,5
AACE recommends that all postmenopausal women 50 years and older be evaluated.1
In addition to patient’s history and physical exam, this guideline recommends that the initial evaluation include a clinical fracture risk assessment with the FRAX™ or other fracture risk assessment tools.1
Bone mineral density testing should also be considered in women age 65 years old or greater, or based on the clinical fracture risk profile.1
When BMD is measured, an axial dual-energy x-ray absorptiometry (DXA) should be used (such as lumbar spine and hip, or 1/3 radius site if indicated).1
The ENDO guidelines recommend all postmenopausal women be evaluated for osteoporosis risk.2
BMD testing should be performed at the lumbar spine and hip, along with a clinical fracture risk assessment with FRAX™. 2
IOF-ESCEO recommends that postmenopausal women who have risk factors for fracture be assessed using the country-specific FRAX™ assessment.5
In patients with intermediate risk, DXA BMD measurements should be included in the FRAX™ calculation.5
Other measurements such as trabecular bone score may also be used in addition to BMD and FRAX™. 5
IOF-ESCEO guidelines further recommend that a vertebral fracture assessment should be considered if the patient has a history of height loss of greater than or equal to 4 cm, kyphosis, or current or recent long-term glucocorticoid therapy, or have a BMD T-score of –2.5 or worse.5
These guidelines provide some similar characterization of fracture risk, categorizing postmenopausal women as “high,” or “very high risk” fracture risk.1-3 The IOF-ESCEO and ENDO Guidelines also include a low fracture risk category, and ENDO additionally includes a moderate Risk category.2,3 We are going to focus on the high and very high risk categories.
Both the AACE and ENDO guidelines classify postmenopausal patients at high risk if they have a history of fracture, a T-score of –2.5 or worse, or an increased fracture risk using FRAX™ country-specific thresholds, shown here for the United States.1,2
Based on guidance from AACE, patients with a recent fracture within the past 12 months, with fractures while on osteoporosis therapy, multiple fractures, fractures while on drugs that cause skeletal harm (such as glucocorticoids), with a very low bone mineral density T-score (such as –3.0 or less), with a history of falls or have high risk for falls, or with a very high fracture probability by FRAX™ (for example, major osteoporosis fracture risk of more than 30%, or hip fracture risk of more than 4.5%) are considered to be at very high fracture risk.1
In the ENDO guidelines, patients are considered very high fracture risk if they have multiple spine fractures and a BMD T-score at the hip or spine of –2.5 or below.2
The IOF and ESCEO recommend that fracture risk is expressed as an absolute risk— probability of fracture over a 10-year interval. It depends on age, life expectancy, and current fracture risk, which is based on clinical risk factors.3
AACE guidelines provide new diagnostic criteria for osteoporosisin postmenopausal women.1
Osteoporosis can be diagnosed if there is a low-trauma (or a fragility) fracture in the absence of other metabolic bone disease, independent of the T-score.1
Individuals with low bone mass, such as a T-score between –1.0 and –2.5, but with a fragility fracture of the spine, hip, proximal humerus, pelvis, or distal forearm are also at a higher risk for future fractures. These individuals should be diagnosed with osteoporosis and pharmacologic therapy should be considered.1
Although traditionally osteoporosis has been diagnosed based on low bone mineral density in the absence of a fracture, the 2020 AACE guidelines state that patients with osteopenia and increased fracture risk using the FRAX™ country-specific thresholds should also be diagnosed with osteoporosis and should be treated.1
The indications for pharmacologic therapy are low T-score, increased fracture risk based on FRAX™, or fragility fracture. Once the diagnosis of osteoporosis is made, the diagnosis remains even if the patient’s T-score improves to better than −2.5 as a result of treatment.1
Guidelines also provide recommendations on who to treat based on history of fracture, FRAX™ fracture risk assessment tool, and T-score.1,2,5
AACE, ENDO, and IOF-ESCEO guidelines recommend that treatment should be provided in patients with a history of fracture. 1,2,5
AACE also includes the criteria of a T-score between –1.0 and –2.5 at the spine, femoral neck, total hip, or 1/3 radius.1
These three guidelines also recommend treatment in patients with increased fracture risk based on country-specific FRAX thresholds. 1,2,5
In the United States, these thresholds are: a FRAX™ 10-year probability of greater than or equal to 20% for major osteoporotic fractures, or greater than or equal to 3% for hip fractures.1,2
The AACE guidelines further specify a T-score between –1.0 and–2.5 in addition to increased fracture risk by FRAX™. 1
Both AACE and ENDO guidelines suggest that patients with a T-score of –2.5 or worse at the lumbar spine, femoral neck, or total hip should receive treatment.1,2
Presented on this slide is a general schema showing AACE recommendations for management of patients at high risk and very high risk.
For those patients who are considered ‘high’ fracture risk, AACE guidelines recommend these patients be started on oral antiresorptive agents.1
The treatment in these ‘high risk’ patients should be monitored by dual-energy X-ray absorptiometry every 1 to 2 years until findings are stable.1
Clinical management depends on BMD and fracture risk category.1
For the patients that fall under the ‘very high’ risk category, AACE guidelines recommend initial treatment options that include anabolic therapy or an injectable antiresorptive.1
Reassessment recommendations are the same as the ‘high’ risk group, with a DXA test every 1 to 2 years as well.1
The guidelines state that an antiresorptive therapy should be used as sequential therapy after anabolic treatment.1
The Endocrine Society also has guidelines for treatment based on fracture risk stratification into ‘high risk’ and ‘very high risk’ categories.2
For those patients who are considered ‘high’ fracture risk, ENDO guidelines recommend initial treatment with antiresorptive agents, which can include bisphosphonates, selective estrogen receptor modulators (or SERMs), menopausal hormone therapy, or calcitonin in selected patients. For example: in women who do not tolerate certain antiresorptive therapies, or in patients with high breast cancer risk, or in women with a hysterectomy and on estrogen-only therapy.2
Patients should be reassessed by dual-energy X-ray absorptiometry every 1 to 3 years.2
For the patients under the ‘very high’ risk category, ENDO guidelines recommend that patients initiate with anabolic therapy for 1 to 2 years.2
In this ‘very high’ risk fracture group, the reassessment recommendations are the same as the ‘high’ risk group, a DXA test every 1 to 3 years.2
The guidelines recommend antiresorptive therapy be used after the completed course of anabolic therapy. This is to help maintain the bone mineral density gains with the initial therapy.2
For these two groups, the ‘high’ risk and ‘very high’ risk categories, the guideline recommends that the therapy continue, or if necessary, switch to another therapy.2
IOF-ESCEO also has an algorithm to risk stratify patients as low risk, high risk, or very high risk of fracture. Treatment recommendations are made based on this categorization.3
In all patients, risk-appropriate exercise and optimizing calcium and vitamin D status are recommended. Antiresorptive therapy is recommended for high risk patients. For very high risk patients, an anabolic agent followed by an inhibitor of bone resorption should be considered.3
In summary, assessment of fracture risk is important to identify individuals at high risk or very high risk for fractures.1-3
There is new criteria for the clinical diagnosis of osteoporosis, and it is based on patient risk factors in addition to bone mineral density.1-3
All three AACE, ENDO, and IOF-ESCEO updated guidelines offer treatment strategy recommendations for high risk and very high risk patients.1-3
Beyond these guidelines, clinicians should familiarize themselves with the tools and technologies available that can help identify patients at high risk that need treatment.
References:
1.Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists/American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr Pract. 2020;26(suppl1):1- 46.This transcript is provided for your convenience and is qualified by the full video in which the spoken content appears. Please review the full video along with the transcript.
Welcome to this video on recent trends in Osteoporosis.
I am Dr. Steven Petak.
This disease awareness program is presented on Amgen’s behalf, and has been reviewed consistent with Amgen’s internal review policies.
Here are my disclosures
I am a member of the Amgen and Alexion speaker bureaus
The incidence of osteoporosis in the United States is projected to increase over the next decade.1
Based on a US Census population, the National Health and Nutrition Examination Survey (NHANES) estimated that approximately 10 million adults in the US who are at least 50 years old had osteoporosis in 2010.1
This is based on a bone mineral density T-score of –2.5 or worse at either the femoral neck or lumbar spine.1
The report projected that the incidence of osteoporosis in this population will increase by 3.4 million in 2030, for an estimated 32% increase.1
The annual number of fractures in women at least 65 years old in the US is also projected to increase in the next two decades.2
In this study, a forecasting model was developed to project the annual incidence of osteoporotic fractures among US women at least 65 years old from 2018 to 2040.2
According to the projected model, the annual number of fractures is expected to increase by 68% by 2040 due to the aging and growing population.2
Despite the projected increase in incidence of osteoporosis that was presented in the previous slide, the rates of osteoporosis diagnosis and the rates of dual-energy x-ray absorptiometry (or DXA) testing declined between 2009 and 2014.3
This is based on a Medicare fee-for-service health claims database that includes women with at least one annual DXA scan.3
The rate of osteoporosis diagnosis was slightly less than 18% in 2009 among women 65 years old or greater.3
This rate decreased to 14.8% in 2014.3
This pattern of decrease was also seen with DXA testing, which was 13.2% in 2008 and decreased to 11.3% in 2014.3
There was a decline in fracture rates between 2007 and 2013.4
Specifically, among women 65 and older, the fracture rate dropped from 27.5 fractures for every 1,000 person-years, down to about 22.1. This represents a significant decrease of 3.4% per year.4
However, in more recent years, the fracture rate has reversed course and has been climbing up again.4
The data points shown in blue on this graph show a relative plateau since 2013, then an increase in fracture rate among women over the age of 65 in 2017.4
Many factors may have played a role in contributing to the increasing fracture rates.1,5
Since 2007, Medicare reimbursement for bone density screening was cut far below the actual cost of performing a dual-energy x-ray absorptiometry scan. As a result, these services were less likely to be offered by physicians in solo practices, and practices with access to fewer than three DXA scanners.5
With the increasing number of fractures over recent years, it is important to recognize that osteoporosis-related fractures can have a negative impact on the patient and can be associated with disability and loss of independence.4
These fractures may prevent a person from caring for him or herself and may require admission to a nursing home or a long-term care facility.6
Costs due to fracture can create a heavy financial burden for the patient and caregivers.7-10
The patient may not be able to do some of the regular activities of daily living because of the fracture.6,11,12
Patients may worry about falls, the possibility of future fractures, and the potential need for nursing home care.8,13
And finally, complications such as chronic pain may occur.11,14
Once a patient experiences a fracture, the risk of having a subsequent fracture increases. This relationship holds true across different sites of initial fracture, including hip, clinical vertebral, upper limb, and lower limb.15
This study published in the Journal of the American Medical Association followed a cohort of ambulatory patients over a 16-year period. These patients lived in the community and not in nursing homes.15
Among elderly women who had a prior fracture, researchers found that the risk of an initial fracture increased with age.15
In addition, the risk for a subsequent fracture went up by 1.6 to 2.4 times.15
In fact, the risk of a subsequent fracture amongst women aged 60–69 years old who had a prior fracture (36%), was higher than the risk of an initial fracture among women aged 70–79 years old (27%).15
The same goes with those in the 70–79 year old group who had a prior fracture. Their risk for a subsequent fracture (63%) was higher than the risk of an initial fracture in those who were 80 years or older (50%).15
This data underscores the fact that the increased risk of a subsequent fracture persists for up to 10 years depending upon age and sex, and independent of initial fracture type.15
When examining the period following an osteoporosis-related fracture, it is evident that the highest risk of a re-fracture is within the first year after the initial fracture.16
This can be illustrated using data from a population-based study of over 4,100 postmenopausal women.16
In this study, fractures included hip fractures, major fractures (including clinical vertebral, forearm, and humerus), and minor fractures (all others), as classified by the World Health Organization.16
The relative risk of a subsequent fracture is 5 times greater in the first year after a fracture compared with the risk of a first fracture. One of every four subsequent fractures happen within the first year after the initial fracture. This further supports early action in prevention of a subsequent fracture.
In summary, osteoporosis-related fractures are common, and the rate has increased, but the rate of diagnosis of osteoporosis has decreased.1-3
The consequences of fractures are significant and can lead to disability and loss of independence, and even more than that, once a person has a fracture, they are more likely to have another fracture. 8,11,15,17
Clinicians should familiarize themselves with the most recent osteoporosis clinical guidelines that provide information that helps to identify and treat patients with osteoporosis.
References:
1. Wright NC, Looker AC, Saag KG, et al. The recent
prevalence of osteoporosis and low bone mass
in the United States based on bone mineral density
at the femoral neck or lumbar spine. J
Bone Miner Res. 2014;29(11):2520-2526.
2. Lewiecki EM, Ortendahl JD, Vanderpuye-Orgle J, et
al. Healthcare policy changes in osteoporosis
can improve outcomes and reduce costs in the United
States. JBMR Plus. 2019;3(9):e10192.
3. Lewiecki EM, Adler R, Curtis J, et al. Hip
fractures and declining DXA testing: at a breaking
point?
Presented at: American Society for Bone and Mineral
Research Annual Meeting. September 16-
19, 2016;Atlanta, GA. Abstract 1077.
4. Lewiecki EM, Chastek B, Sundquist K, et al.
Osteoporotic fracture trends in a population of US
managed care enrollees from 2007 to 2017. Osteoporos
Int. 2020;31(7):1299-1304
5. Hayes BL, Curtis JR, Laster A, et al.
Osteoporosis care in the United States after
declines in
reimbursements for DXA. J Clin Densitom.
2010;13(4):352-360.
6.Bentler SE, Liu L, Obrizan M, et al. The aftermath
of hip fracture: discharge placement,
functional status change, and mortality. Am J
Epidemiol. 2009;170(10):1290-1299.
7.Tajeu GS, Delzell E, Smith W, et al. Death,
debility, and destitution following hip fracture. J
Gerontol A Biol Sci Med Sci. 2014;69(3):346-353.
8.National Osteoporosis Society. Life with
Osteoporosis. October 2014.https://www.laterlifetr
aining.co.uk/wp-content/uploads/2014/10/NOS-Public-Report-V2.2-Small-file-size.pdf.
Accessed
February 4, 2021.
9.Tarride J-E, Hopkins RB, Leslie WD, et al. The
burden of illness of osteoporosis in Canada.
Osteoporos Int. 2012;23(11):2591-2600.
10.Kaffashian S, Raina P, Oremus M, et al. The
burden of osteoporotic fractures beyond acute care:
the Canadian Multicentre Osteoporosis Study (CaMos).
Age Ageing. 2011;40(5):602-607.
11. Cosman F, de Beur SJ, LeBoff MS, et al.
Clinician's guide to prevention and treatment of
osteoporosis. Osteoporos Int. 2014;25(10):2359-2381.
12.Fischer S, Kapinos KA, Mulcahy A, Pinto L, Hayden
O, Barron R. Estimating the long-term
functional burden of osteoporosis-related fractures.
Osteoporos Int. 2017;28(10):2843-2851.
13. Vass CD, Sahota O, Angelova T. Fear of falling
after fragility fracture–a prevalence study. Age
Ageing. 2014;43:i29.
14.Inacio MCS, Weiss JM, Miric A, Hunt JJ, Zohman
GL, Paxton EW. A community-based hip fracture
registry: population, methods, and outcomes. Perm J.
2015;19(3):29-36.
15.Center JR, Bliuc D, Nguyen TV, Eisman JA. Risk of
subsequent fracture after low-trauma fracture
in men and women. JAMA. 2007;297(4):387-394.
16.van Geel TACM, van Helden S, Geusens PP, Winkens
B, Dinant G-J. Clinical subsequent fractures
cluster in time after first fractures. Ann Rheum
Dis. 2009;68(1):99-102.
17.Camacho PM, Petak SM, Binkley N, et al. American
Association of Clinical
Endocrinologists/American College of Endocrinology
clinical practice guidelines for the diagnosis
and treatment of postmenopausal osteoporosis-2020
update. Endocr Pract. 2020;26(suppl1):1-
46.
This transcript is provided for your convenience and is qualified by the full video in which the spoken content appears. Please review the full video along with the transcript.
Once you experience a fracture due to osteoporosis, the risk of having another fracture goes up. A first fracture represents an opportunity to intervene with appropriate treatment to reduce the risk of a future fracture. Post-fracture care, or PFC, is a collaborative approach that helps patients who have experienced a fracture avoid subsequent fractures.
PFC involves a team of specialists, primary care and ER physicians, nurses, and other health care staff, who all work together to decrease the risk of the patient fracturing again.
PFC programs are important because they improve patient follow-up, testing, and treatment for osteoporosis.
The percentages of patients who received follow-up, testing, and treatment were significantly higher after PFC than before PFC.
Primary care practitioners have a key role in PFC programs.
When receiving patients from PFC, primary care practitioners should maintain appropriate communication with patients, other physicians, and PFC team members maintain the patient’s treatment plan continue to conduct bone health assessments and continue to evaluate risk factors for fracture.
The most important thing you can do for your patients is to identify those with fragility fractures, regardless of when the fracture occurred. PFC programs work best when primary care practitioners conduct an assessment of bone health, risk factors for fracture, and suitability of treatment and nonpharmacologic interventions.
Lack of adherence to osteoporosis medication correlates with an elevated risk of fracture. To help avoid future fractures, maintain communication to assess treatment adherence coordinate treatment with other healthcare providers and continue to monitor for fragility fractures and other risk factors.
This transcript is provided for your convenience and is qualified by the full video in which the spoken content appears. Please review the full video along with the transcript.
Among the many conditions seen in everyday clinical practice, including breast cancer, myocardial infarction, and stroke, fragility fractures represent a substantial health burden.
1 in 3 women, 50 years of age or older, will suffer fragility fractures worldwide.
Fragility fractures are warning signs of osteoporosis and are a growing global problem. For example, a 240% global increase in hip fractures among women is projected by 2050 compared to rates in 1990. Hip fractures can lead to disability and loss of independence.
However, fewer than 1 in 5 patients with osteoporosis receive a diagnosis. And
fewer than 1 in 3 patients receive treatment, even after a fracture.
The diagnosis of osteoporosis may be missed if signs and symptoms are similar to those of common agerelated conditions. Screen all postmenopausal women 50 years of age or older with risk factors and have a conversation about their bone health.
References:
1. Rosamond W, et al. Circulation.
2008;117:e25-e146.
2. American Cancer Society.
www.cancer.org/content/dam/cancer-org/research/
cancer-factsandstatistics/
annual-cancer-facts-and-figures/2005cancer-facts-andfigures-2005.
pdf. Accessed
October 15, 2019.
3. Burge R, et al. J Bone Miner Res.
2007;22:465-475.
4. International Osteoporosis Foundation.
www.iofbonehealth.org/facts-statistics. Accessed
October 15, 2019.
5. Gullberg B, et al. Osteoporos Int.
1997;7:407-413.
6. Camacho PM, et al. Endocr Pract. 2016;22(suppl
4):1-42.
7. International Osteoporosis Foundation.
www.capturethefracture.org/slide-kits-reports.
Accessed October 15, 2019.
8. Yusuf AA, et al. Arch Osteoporos. 2016;11:31.
9. Vondracek SF, Linnebur SA. Clin Interv Aging.
2009;4:121-136.
10. Cosman F, et al. Osteoporos Int.
2014;25:2359-2381.
This transcript is provided for your convenience and is qualified by the full video in which the spoken content appears. Please review the full video along with the transcript.
Hello, and welcome to the Osteoporosis Clinical Insight video series. I am Dr. Nelson Watts, and I will be your guide.
in this series of four short videos exploring bone anatomy and physiology, fracture risk assessment, and diagnosis of osteoporosis, as well as the use of DXA measurement of bone mineral density in clinical management.
Osteoporosis is a systemic skeletal disease defined by low bone mass, microarchitectural deterioration of bone tissue, and the resulting increased bone fragility and susceptibility to fracture.1 In this first video, we will examine the normal architecture of bone and the bone remodeling cycle, and discuss the changes that lead to osteoporosis.
So, to begin. Bone is a dynamic tissue that is the primary constituent of the skeleton, serving as a scaffold for muscles, a reservoir for minerals such as calcium and phosphorus, the location for production of blood cells, and protection of internal organs. 2
For the purpose of understanding osteoporosis, bone can be classified according to anatomy and microarchitecture. 2
Anatomically, the skeleton is divided into the axial or central skeleton, which includes the skull, spine, sternum, ribs, and sacrum; and the appendicular or peripheral skeleton, which is made up of the clavicles, scapulae, pelvis, and the bones of the arms and legs. 2
Microarchitecturally, bone tissue can be classified as cortical or trabecular. 2 Cortical bone is the dense outer shell. It is solid but interspersed with vascular channels.3 Cortical bone has high resistance to torsion.3 Cortical bone becomes more porous with age or disease, which leads to a loss of strength. 4
Trabecular bone - also called cancellous or spongy bone - is the inner, less dense and more elastic part of the bone, which provides resistance to compressive forces.2 It is composed of a honeycomb-like lattice,2 and accounts for approximately 20% of total bone mass.4 The struts and plates that make up trabecular bone are arranged in patterns that provide maximal strength and mechanical support.3 Trabecular bone has a large surface area, a high turnover rate, is more metabolically active and is preferentially affected by osteoporosis.3
Every bone in the skeleton contains both cortical and trabecular components, although the proportion varies.3 Cortical bone predominates in the appendicular or peripheral skeleton, as bones there need to resist both torsional and compressive forces. Conversely, trabecular bone predominates in the axial or central skeleton, particularly the vertebrae, which are subjected mostly to compressive forces.2,3 Compressive fractures of the vertebrae are classic osteoporotic fractures.4 Other common fracture sites include the wrist and the hip, but osteoporotic fractures can occur in other bones as well.2
Now that we have a better understanding of bone structure, let’s discuss how bone is built and maintained during a person’s lifespan. We call these processes bone modelling and remodelling, respectively.3 Remodeling includes removal and replacement of bone; modeling is bone formation that is not preceded by resorption.3 Modeling is the main process in childhood and adolescence; more bone is formed than is removed and bones change in size and shape.2 Bone mass is at or near its peak by the third decade of life in both men and women.5 In early adulthood, there is a balance between bone formation and resorption.3 Later in life, as shown in this graph, the balance shifts towards resorption, leading to age-related bone density loss. In women, menopause significantly tips the balance more toward resorption.2
At the cellular level, the bone remodeling cycle occurs in several distinct steps.2 An activation signal originates from osteocytes, cells embedded in the bone matrix.2 Stromal cells and other cells in the bone microenvironment release factors that induce precursor cells in the bone marrow to differentiate into osteoclasts, which resorb bone.2 After the resorption phase ends, osteoclasts move away, clearing the way for osteoblasts, whose differentiation and activation is mediated by other molecular signals. Osteoblasts produce new bone matrix and promote its mineralization.2 Finally, remaining osteoblasts differentiate into bone lining cells and new osteocytes.2
Now that we have reviewed the normal physiology of bone, we can understand the pathogenesis of osteoporosis. It typically results from one or more of the following four processes:
First: Failure to achieve an optimal peak bone mass, due to genetic factors, a sedentary lifestyle, or a calcium or vitamin D deficiency.2
Second: Accelerated bone resorption later in life. This can result from estrogen deficiency in peri- and postmenopausal women, calcium or vitamin D deficiency, long-term glucocorticoid use, and increased inflammatory cytokine activity.2
Third: Impaired bone formation, which also results in net bone loss, and can occur because of chronic glucocorticoid treatment, and fourth and finally:2 Poor bone quality, including the presence of microfractures - which can initiate larger fractures - the arrangement and the amount of crosslinking of bone matrix collagen, as well as the ratio of mineral to matrix in the bone.2
This concludes the first video in the Osteoporosis Clinical Insight series. Next time, we will take a look at osteoporosis screening and fracture risk assessment.
References:
1. Compston JE, McClung MR, Leslie WD. Osteoporosis.
Lancet. 2019 Jan 26;393(10169):364-376.
2. Saag K, Morgan SL, Clines GA. Diagnosis and
Management of Osteoporosis. 3rd ed.
Professional
Communications, Inc; 2019.
3. Kenkre JS, Bassett J. The bone remodelling cycle.
Ann
Clin Biochem. 2018 May;55(3):308-327.
4. Ott SM. Cortical or Trabecular Bone: What's the
Difference? Am J Nephrol. 2018;47(6):373-375.
5. Schtscherbyna A, Ribeiro BG, and Fleiuss FML.
Bone
health, bone mineral density, and sports
performance. In Nutrition and enhanced sports
performance, 73–81. Academic Press; 2019.
6. Canalis E, Giustina A, Bilezikian JP. Mechanisms
of
anabolic therapies for osteoporosis. N Engl J
Med. 2007 Aug 30;357(9):905-16.
This transcript is provided for your convenience and is qualified by the full video in which the spoken content appears. Please review the full video along with the transcript.
Hello, and welcome to the second video in the Osteoporosis Clinical Insight series. I am Dr. Nelson Watts, and I will be your guide as we discuss osteoporosis risk factors, screening for osteoporosis, and fracture risk assessment. In the last video, we saw that osteoporosis is defined by increased bone fragility as a consequence of low bone mass and deterioration of bone microarchitecture.1
Research has revealed many risk factors that increase a person’s chance of developing osteoporosis. These can be grouped into three categories. First, there are modifiable lifestyle risk factors, including smoking, excessive alcohol use (3 or more units of alcohol a day),2,3 deficiency of vitamin D or calcium, and a low body mass index.4 Then there are unmodifiable risk factors, of which genetics is a large component. For example, a parental history of hip fracture is an unmodifiable genetic risk factor.4 Finally, hormonal factors, including menopause, also contribute to osteoporosis risk.4
But how do we assess fracture risk in individual patients? A key parameter is bone mineral density, or BMD, which is an important component of bone strength.4 By far the most widely-used BMD assessment method is dual-energy X-ray absorptiometry, or DXA. DXA uses low-radiation X-rays of two different wavelengths to measure bone mineral content in a given area. The output is BMD in grams per centimeter squared.4 We will take a more detailed look at BMD using DXA in the final video of the series.
Other methods may also be used to assess BMD in certain contexts, such as in peripheral skeletal sites, like the heel, radius, or tibia. These include quantitative ultrasound, which requires only small and portable devices, and doesn’t use ionized radiation.4 Another is quantitative computed tomography, or QCT, which is more expensive than DXA4 and has higher radiation exposure than DXA,4 but measures volumetric BMD rather than areal BMD measured by DXA,5 and is independent of body size; it can be used to measure BMD in the spine and hip in addition to peripheral sites.4 High-resolution peripheral QCT, or HR-pQCT, may be used to assess bone microarchitecture in the distal tibia and distal radius, though it requires a special instrument.5 Finally, it should be mentioned that previously acquired CT images can be used to determine BMD - a practice known as “opportunistic screening”.1
Although BMD is an important parameter in fracture risk assessment, it only accounts for approximately 60 to 70% of bone strength. Other bone properties, referred to collectively as “bone quality” also contribute to bone strength.
These properties include deterioration of bone microarchitecture,6 microfractures, collagen fiber disorganization, reduced collagen crosslinking, and lower mineral to matrix ratio in the bone.4 The need to quantify microarchitectural deterioration led to the development of the trabecular bone score, or TBS, which is a textural measurement derived from lumbar spine DXA images that serves as an index of bone microarchitecture. TBS correlates with fracture risk independent of BMD.6 Bone turnover markers measured in serum may be associated with bone loss, increased fracture risk, and poor treatment adherence; however, their role in clinical practice is presently unclear.4
Once BMD and perhaps TBS values have been obtained, how are they combined into an overall expression of fracture risk? One answer to this question are fracture risk calculators, which have been developed for this purpose.5 The most well-studied calculator is FRAX.5 FRAX is a computer-based algorithm, available on the Web and part of many bone density reporting systems, that calculates fracture probability in untreated patients from easily-obtained clinical risk factors. FRAX can be done with or without the input of femoral neck BMD. The output of FRAX is the 10-year probability of a major osteoporotic fracture - hip, clinical spine, humerus, or wrist fracture - as well as the 10-year probability of hip fracture.7 FRAX can include the TBS score, which improves its predictive value.4 Low TBS scores increase FRAX-estimated risk, while high TBS values reduce it.8
Now that we have briefly surveyed the methods and tools of bone quality and fracture risk assessment, we may ask: How are they practically employed in screening for osteoporosis? The International Society for Clinical Densitometry, or ISCD, is a professional society which periodically publishes recommendations based on evidence and expert opinion.9 The latest set of ISCD recommendations was released in 2019 and includes a list of indications for BMD testing.
It is important to stress that everyone should undergo BMD testing eventually; the ISCD provides consensus criteria on when to do so.9
BMD testing is indicated for women 65 and older9 and for postmenopausal women aged younger than 65 if they have a risk factor for low bone mass, such as a family history of osteoporosis, low body weight, prior fracture, use of high-risk medications, or have a condition associated with bone loss. It is also indicated for women during menopausal transition who have one or more clinical risk factors for fracture.9
BMD testing is indicated for all men aged 70 and older, and for those less than 70 years old if they have at least one risk factor for low bone mass.9 So, everyone should have a bone density test eventually: women by age 65, men by age 70. Younger men and postmenopausal women who have experienced a fracture due to skeletal fragility, or have a disease or condition associated with bone loss, or take medication associated with low bone mass or bone loss should be tested earlier.9
That concludes this overview of osteoporosis risk assessment and screening. In the next video, we will see how the techniques we have learned about so far come into play when making a formal diagnosis of osteoporosis, and in post-diagnostic evaluation of the patient.
References:
1. Compston JE, McClung MR, Leslie WD. Osteoporosis.
Lancet. 2019 Jan 26;393(10169):364-376.
2. Camacho PM, Petak SM, Binkley N, et al. AMERICAN
ASSOCIATION OF CLINICAL
ENDOCRINOLOGISTS/AMERICAN COLLEGE OF ENDOCRINOLOGY
CLINICAL PRACTICE
GUIDELINES FOR THE DIAGNOSIS AND TREATMENT OF
POSTMENOPAUSAL OSTEOPOROSIS-
2020 UPDATE. Endocr Pract. 2020 May;26(Suppl
1):1-46.
3. Kanis JA, Johansson H, Johnell O, et al. Alcohol
intake as a risk factor for fracture. Osteoporos
Int. 2005 Jul;16(7):737-42.
4. Saag K, Morgan SL, Clines GA. Diagnosis and
Management of Osteoporosis. 3rd ed.
Professional
Communications, Inc; 2019.
5. Leder BZ, Wein, MN (eds). Osteoporosis
Pathophysiology and Clinical Management. 3rd
ed.
Humana Press; 2020.
6. Martineau P, Silva BC, Leslie WD. Utility of
trabecular bone score in the evaluation of
osteoporosis. Curr Opin Endocrinol Diabetes Obes.
2017
Dec;24(6):402-410.
7. Watts NB. The Fracture Risk Assessment Tool
(FRAX®):
applications in clinical practice.
J Womens Health (Larchmt). 2011
Apr;20(4):525-31.
8. Binkley N, Leslie WD. Clinical Application of
Spine
Trabecular Bone Score (TBS). Clinic Rev Bone
Miner Metab. 2016;14:14-24.
9. International Society for Clinical Densitometry
(2019). 2019 ISCD Official Positions - Adult.
Available at:
https://iscd.org/learn/official-positions/adult-positions/.
This transcript is provided for your convenience and is qualified by the full video in which the spoken content appears. Please review the full video along with the transcript.
Hello, and welcome to the third video in the Osteoporosis Clinical Insight series. I am Dr. Nelson Watts, and I will be your guide as we discuss how osteoporosis is diagnosed, and how it is evaluated following diagnosis.
The initial evaluation for osteoporosis should include three elements. First, a detailed medical history is needed,1 which may reveal risk factors that we discussed in the last video, such as parental history of hip fracture.2 At this stage, FRAX or another clinical risk assessment tool may be used, which is typical for countries outside the United States.1 Second, a physical examination should be performed.1 This may reveal kyphosis or height loss, which are clues of vertebral fractures, or scoliosis, leg length disparity or muscle weakness, which may indicate an increased risk of falling.2 Finally, the patient’s clinical risk factor profile informs whether BMD should be measured.1 In the US, FRAX risk assessment is typically performed including the femoral neck BMD results, as DXA instruments usually come with integrated FRAX software.3
As we saw in the last video, DXA is the preferred method to assess BMD in patients suspected of having osteoporosis. DXA-measured BMD of sites of particular interest - the lumbar spine, femoral neck, and total hip - is obtained for diagnosis.1
BMD is determined – in grams per centimeter squared – and then a T-score is calculated by comparing the patient’s value with the average young adult mean value. The T-score quantifies the difference between the patient’s BMD and that of a reference population and the difference is expressed as a standard deviation score.2 The risk of fracture increases approximately two-fold for each standard deviation decrease in BMD, such that a T-score of -1.0 represents a two-fold increase, -2.0 is a four-fold increase, and -3.0 is an eight-fold increase in fracture risk.4
How is this information applied and a formal diagnosis of osteoporosis made? In 2020, the American Association of Clinical Endocrinologists, AACE, published an update to their 2016 Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis. The updated guidelines include four situations in which a diagnosis of osteoporosis is warranted.1
First, a T-score of -2.5 or below in the lumbar spine, femoral neck, total hip, or one-third radius. Second, the occurrence of a low-trauma fracture in the spine or hip, regardless of BMD. Third, a T-score between -1.0 and -2.5 in addition to a fragility fracture in the proximal humerus, pelvis, or distal forearm. And finally, a T-score between -1.0 and -2.5 in addition to high fracture probability as assessed by FRAX according to country-specific thresholds. Osteoporosis is diagnosed if any of these conditions is met.1
Once osteoporosis is diagnosed, further evaluation is warranted to inform treatment, disease management, and follow-up. Several factors may point to a very high risk of fracture. Examples include the occurrence of fractures in the previous 12 months, multiple fractures, fractures during approved therapy for osteoporosis or while on medications associated with bone loss such as glucocorticoids, very low T-scores, increased fall risk or a history of falls, and very high FRAX-calculated fracture probability.1
Beyond stratification, disease assessment following diagnosis should also include an evaluation for contributing factors, as bone loss may be caused or exacerbated by coexisting medical conditions which may be asymptomatic and require laboratory testing for identification.1 Appropriate post-diagnostic evaluation may also include assessing for vertebral fractures, which indicate a higher risk for future fractures.1 In the next video, we will discuss imaging techniques used to look for the presence of vertebral fractures. Finally, the post-diagnostic assessment may include the measurement of bone turnover markers, including osteoblast-derived factors and metabolic by-products of bone resorption, which can predict higher rates of bone loss.1
That concludes our overview of how osteoporosis is diagnosed. Next time, we conclude the series with a deeper dive into how DXA scans are acquired, and how to interpret DXA studies.
References:
1. Camacho PM, Petak SM, Binkley N, et al. American
Association of Clinical
Endocrinologists/American College of Endocrinology
Clinical Practice Guidelines for the
Diagnosis and Treatment of Postmenopausal
Osteoporosis-2020 Update. Endocr Pract. 2020
May;26(Suppl 1):1-46.
2. Saag K, Morgan SL, Clines GA. Diagnosis and
Management of Osteoporosis. 3rd ed.
Professional
Communications, Inc; 2019.
3. Watts NB. The Fracture Risk Assessment Tool
(FRAX®):
applications in clinical practice.
J Womens Health (Larchmt). 2011
Apr;20(4):525-31.
4. Leder BZ, Wein, MN (eds). Osteoporosis
Pathophysiology and Clinical Management. 3rd
ed.
Humana Press; 2020.
This transcript is provided for your convenience and is qualified by the full video in which the spoken content appears. Please review the full video along with the transcript.
Hello, and welcome to the fourth and final video in the Osteoporosis Clinical Insight series. I am Dr. Nelson Watts, and I will be your guide as we take a deeper dive into the topic of DXA measurement for bone mineral density or BMD. We will discuss common pitfalls in DXA acquisition, the best practices to minimize such errors, and what constitutes a good report. Finally, we will look at the use of DXA-based equipment to identify vertebral fractures.
We saw in earlier videos that DXA is the standard for assessing BMD.1 As such, it is a useful tool to evaluate fracture risk,2 to formally diagnose osteoporosis,3 and to follow the response to treatment.3 Remember that we cannot rely solely on DXA-derived T-scores to diagnose osteoporosis and choose treatment. As we have seen in previous videos, osteoporosis may be diagnosed in patients with a T-score better than -2.5 who have had a fracture or high fracture risk determined with FRAX.3
Now, let’s look at some of the errors that can happen during DXA acquisition and interpretation. The quality of a DXA study depends on the skill and experience of the technologist who positions the patient and acquires and analyzes the images and on the clinician who finalizes the report.2 Mistakes are surprisingly common. One study in Italy found that up to 90% of DXA studies and reports contained at least one mistake.2 The most common errors were in data acquisition, incorrect patient positioning, and radiographic artifacts.2
To minimize such errors, best practices have been developed. For example, the International Society for Clinical Densitometry (ISCD) has established a set of minimum requirements that it recommends be followed during DXA measurement.2
The minimum reporting requirements include information about the requesting physician and the indication for testing; patient details, including demographics and fracture risk factors; machine specifics; numerical BMD and T-score values; statements about the technical quality of the scan and a recommendation concerning another scan in the future. Where appropriate, the report can also contain a statement on fracture risk or the need to evaluate for causes of secondary osteoporosis.2
In addition to the recommendations for reporting, there are several other best practices to follow. There should be only one diagnosis for each patient rather than a diagnosis for each site imaged, and, if applicable, the results of a fracture risk assessment tool such as FRAX may be included.2,4 Lastly, we should mention that serial BMD studies may be performed to monitor changes over time. In such cases, it is best that serial measurements be performed on the same scanner as the initial test. Differences in BMD should only be reported as changes if they meet or exceed the least significant change calculated using the ISCD-developed formula.4,5
When these best practices are implemented and pitfalls avoided, a high-quality DXA study like the one on the right can maximally inform the patient’s primary healthcare providers.
Finally, let’s examine the role of DXA equipment in vertebral fracture assessment. As you may recall, checking for prevalent vertebral fractures is important because vertebral fractures are important indicators of future fracture6 but often missed.3
The two most useful methods include lateral radiographs of the thoracic and lumbar spine, as well as vertebral fracture assessment - or VFA - done with DXA equipment.3
How do we decide which patient needs a VFA? The current AACE guidelines recommend that a VFA be done in patients with a T-score below -1.0 who fall into one of the following four categories: First, being 70 or older for women or 80 or above for men, Second, height loss of more than 4 centimeters; Third, a history of a prior vertebral fracture that is not otherwise documented, and Fourth, receiving glucocorticoid therapy equivalent to prednisone 5 mg a day or more for 3 months or longer.3
How do the two methods for vertebral fracture detection compare? The lateral spine X-ray provides higher image quality.7 However, VFA has several advantages, including a lower radiation dose, lower cost, and the convenience of assessing for vertebral fractures at the same time as measuring BMD.7 The image on the right shows a VFA radiograph that was used to identify a fracture in the T9 vertebra.8 The emerging consensus is that VFA is as useful in detecting vertebral fractures as lateral spine radiographs.7
That concludes our discussion of some of the most important principles of DXA scan acquisition and reporting, and the Osteoporosis Clinical Insights series as a whole. We hope you have enjoyed the series!
References:
1. Leder BZ, Wein MN. Osteoporosis
Pathophysiology and
Clinical Management. 3rd ed. Humana
Press; 2020.
2. Saag K, Morgan SL, Clines GA. Diagnosis and
Management of Osteoporosis. 3rd ed.
Professional
Communications, Inc; 2019.
3. Camacho PM, Petak SM, Binkley N, et al. AMERICAN
ASSOCIATION OF CLINICAL
ENDOCRINOLOGISTS/AMERICAN COLLEGE OF ENDOCRINOLOGY
CLINICAL PRACTICE
GUIDELINES FOR THE DIAGNOSIS AND TREATMENT OF
POSTMENOPAUSAL OSTEOPOROSIS-
2020 UPDATE. Endocr Pract. 2020 May;26(Suppl
1):1-46.
4. Lewiecki EM, Binkley N, Morgan SL, et al;
International Society for Clinical Densitometry.
Best
Practices for Dual-Energy X-ray Absorptiometry
Measurement and Reporting: International
Society for Clinical Densitometry Guidance. J
Clin
Densitom. 2016 Apr-Jun;19(2):127-40.
5. Shepherd JA, Lu Y. A generalized least
significant
change for individuals measured on different
DXA systems. J Clin Densitom. 2007
Jul-Sep;10(3):249-58.
6. Malgo F, Hamdy NAT, Ticheler CHJM, et al. Value
and
potential limitations of vertebral fracture
assessment (VFA) compared to conventional spine
radiography: experience from a fracture
liaison service (FLS) and a meta-analysis. Osteoporos
Int. 2017 Oct;28(10):2955-2965.
7. Oh SH, Kim D, Lee YE, et al. Comparison of
screening
strategies for prevalent vertebral fractures
in South Korea: vertebral fracture assessment vs.
spine
radiography. BMC Musculoskelet
Disord. 2018 Feb 12;19(1):46.
8. Guerri S, Mercatelli D, Aparisi Gómez MP, et al.
Quantitative imaging techniques for the
assessment of osteoporosis and sarcopenia. Quant
Imaging
Med Surg. 2018;8(1):60-85.
This transcript is provided for your convenience and is qualified by the full video in which the spoken content appears. Please review the full video along with the transcript.
Accidents happen.
But after a simple fall from standing height,1,2 the difference between a momentary mishap and a fracture may be more than an accident3-5 and is potentially a result of compromised bone strength.3-5 A fragility fracture, also often referred to as a low-trauma fracture,1,5,6 could reflect a deficit in bone mass and structural integrity,5,7 the main determinants of bone strength.8 This deficit occurs when bone formation by osteoblasts fails to counterbalance bone resorption by osteoclasts.7 Every three seconds, someone in the world experiences a low-trauma clinical fracture,9 a fragility fracture that causes immediate pain and disability.6,10 Clinical fractures happen anywhere in the skeleton and include nonvertebral2,11 and symptomatic vertebral fractures.9 These fractures can result in a substantial burden to individuals and society.10 Any such fracture signals increased risk for a subsequent clinical fracture.2 After a clinical fracture, one in 4 women will sustain another fracture in the next 5 years.12 A clinical fracture signals a need for immediate action to reduce the risk of further fractures.5 Improving bone mass, structure, and strength can help protect against further fractures.5,7
The skeleton is a dynamic organ with a capacity to change its mass and structure7,13 by way of multiple signaling pathways that regulate bone formation and resorption.13-15 Extensive crosstalk among osteocytes, osteoblasts, and osteoclasts affects signaling via these pathways.14 Osteocytes can limit bone formation by secreting Wnt antagonists.16 Of these, sclerostin is a key negative regulator of bone formation in adults.14,17 Under conditions of reduced weight bearing18 or postmenopausal estrogen deficiency,19,20 osteocytes secrete more sclerostin. At the cellular level, sclerostin interferes with Wnt coreceptor signaling,18,21 thus reducing the amount of new bone being formed by osteoblasts.18 Sclerostin also increases osteoclast formation and resorptive activity indirectly by increasing the expression of RANKL and decreasing the expression of OPG in osteoblast lineage cells.22 Under conditions of mechanical loading,as in exercise23,24 or with PTH19,23 and estrogen signaling,19,20 osteocytes secrete less sclerostin, allowing Wnt to bind to its coreceptors,16 resulting in signaling associated with increased bone formation by osteoblasts.16 These responses are part of the body’s diverse repertoire for regulating bone mass.14
References
1. Prentice A, Schoenmakers I, Laskey MA, de Bono S, Ginty F, Goldberg GR. Nutrition and bone
growth and development. Proc Nutr Soc. 2006;65:348-60.
2. Center JR, Bliuc D, Nguyen TV, Eisman JA. Risk of subsequent fracture after low-trauma
fracture in men and women. JAMA. 2007;297:387-94.
3. Bouxsein ML, Seeman E. Quantifying the material and structural determinants of bone strength.
Best Pract Res Clin Rheumatol. 2009;23:741-53.
4. Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects.
J Clin
Invest.
2005;115:3318-25.
5. Boonen S, Singer AJ. Osteoporosis management: impact of fracture type on cost and quality of
life in patients at risk for fracture I. Curr Med Res Opin. 2008;24:1781-8.
6. Abimanyi-Ochom J, Watts JJ, Borgström F, et al. Changes in quality of life associated with
fragility fractures: Australian arm of the International Cost and Utility Related to
Osteoporotic Fractures Study (AusICUROS). Osteoporos Int. 2015;26:1781-90.
7. Seeman E, Delmas PD. Bone quality: the material and structural basis of bone strength and
fragility. N Engl J Med. 2006;354:2250-61.
8. Seeman E. Bone quality: the material and structural basis of bone strength.
J Bone Miner
Metab.
2008;26:1-8.
9. Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with
osteoporotic fractures. Osteoporos Int. 2006;17:1726-33.
10. U.S. Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the
Surgeon General. Rockville, MD: U.S. Department of Health and Human Services, Office of the
Surgeon General, 2004.
11. van Helden S, Cals J, Kessels F, Brink P, Dinant GJ, Geusens P. Risk of new clinical
fractures within 2 years following a fracture. Osteoporos Int. 2006;17:348-54.
12. Bliuc D, Nguyen ND, Nguyen TV, Eisman JA, Center JR. Compound risk of high mortality
following osteoporotic fracture and refracture in elderly women and men.
J Bone Miner
Res.
2013;28:2317-24.
13. Raggatt LJ, Partridge NC. Cellular and molecular mechanisms of bone remodeling.
J Biol
Chem.
2010;285:25103-8.
14. Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to
treatments. Nat Med. 2013;19:179-92.
15. Blau JE, Collins MT. The PTH-Vitamin D-FGF23 axis. Rev Endocr Metab Disord.
2015;16:165-74.
16. Robling AG, Niziolek PJ, Baldridge LA, et al. Mechanical stimulation of bone in vivo reduces
osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866-75.
17. van Bezooijen RL, Roelen BA, Visser A, et al. Sclerostin is an osteocyte-expressed negative
regulator of bone formation, but not a classical BMP antagonist. J Exp Med.
2004;199:805-14.
18. Lin C, Jiang X, Dai Z, et al. Sclerostin mediates bone response to mechanical unloading
through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res. 2009;24:1651-61.
19. Mirza FS, Padhi ID, Raisz LG, Lorenzo JA. Serum sclerostin levels negatively correlate with
parathyroid hormone levels and free estrogen index in postmenopausal women.
J Clin Endocrinol
Metab.
2010;95:1991-7.
20. Jia HB, Ma JX, Ma XL, et al. Estrogen alone or in combination with parathyroid hormone can
decrease vertebral MEF2 and sclerostin expression and increase vertebral bone mass in
ovariectomized rats. Osteoporos Int. 2014;25:2743-54.
21. Li X, Zhang Y, Kang H, et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt
signaling. J Biol Chem. 2005;280:19883-7.
22. Wijenayaka AR, Kogawa M, Lim HP, Bonewald LF, Findlay DM, Atkins GJ. Sclerostin stimulates
osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS One.
2011;6(10):e25900.
23. Ke HZ, Richards WG, Li X, Ominsky MS. Sclerostin and Dickkopf-1 as therapeutic targets in
bone diseases. Endocr Rev. 2012;33:747-83.
24. Kontulainen S, Sievänen H, Kannus P, Pasanen M, Vuori I. Effect of long-term impact- loading
on mass, size, and estimated strength of humerus and radius of female racquet- sports players: a
peripheral quantitative computed tomography study between young and old starters and controls.
J Bone Miner Res. 2002;17:2281-9.

This transcript is provided for your convenience and is qualified by the full video in which the spoken content appears. Please review the full video along with the transcript.
Discoveries in bone biology have revealed the role of the RANK ligand pathway in osteoclast-mediated bone loss and postmenopausal osteoporosis.1,2
Osteoporosis is a significant health burden, compromising the strength of bones and increasing the risk for fracture.3
Menopause is a key turning point in the skeletal health of women.3,4
Following menopause, declines in estrogen often lead to excessive bone remodeling activity and accelerated bone loss.3-5
Bone loss following menopause results from an imbalance of osteoclast and osteoblast activity.4,6-8
Osteoclasts are the specialized cells that resorb bone, and osteoblasts are the cells that form new bone.6,8
The discovery of the RANK ligand pathway has been an important advance in our understanding of bone remodeling.1
RANK ligand, a protein expressed by osteoblasts, plays a key role in osteoclast formation, function, and survival through interaction with its receptor, RANK, that is expressed on the surface of osteoclasts.1
Osteoprotegerin, or OPG, another protein secreted by osteoblasts, is a natural inhibitor of RANK ligand and plays a role in regulating bone resorption.1
At the initiation of bone remodeling, lining cells move apart to expose the bone surface, become osteoblasts, and begin expressing RANK ligand.8-10
RANK ligand binds to RANK on osteoclast precursors, which initiates cell fusion and the formation of mature, multinucleated osteoclasts.1,2
RANK ligand continues to bind to RANK on mature osteoclasts.2
The binding of RANK ligand to RANK is essential for osteoclast formation, function, and survival.1,2
Following bone resorption, osteoblasts migrate into the pit.10
Osteoblasts fill the pit with new bone matrix.8,10
Some osteoblasts become embedded within the matrix and eventually turn into osteocytes, while others become new lining cells on the bone surface.10
In the final stage of remodeling, newly created bone matrix mineralizes and the bone returns to a resting state.8,10
The process of bone remodeling is regulated by factors including estrogen and OPG.2,5,11
Estrogen limits the amount of RANK ligand expression by osteoblasts and OPG blocks the binding of RANK ligand to RANK, thereby reducing osteoclast activity.1,2,11
In postmenopausal women, reduced levels of estrogen lead to increased expression of RANK ligand by osteoblasts.11
Excessive RANK ligand overwhelms OPG,1,3 leading to more osteoclasts, increased bone remodeling activity, and greater bone loss.1,2
Osteoblasts continue to deposit new bone matrix, but they can not replace all of the resorbed bone.5,6 Therefore, resorption pits may not be completely refilled, which over time leads to thinning and weakening of bone.3,5,6
The progressive loss of bone following menopause reduces the structural integrity and strength of the skeleton.3,6,7,12
Bone loss may go undetected for many years until the occurrence of a fracture, a potentially serious and debilitating outcome of postmenopausal osteoporosis.13,14
In summary, in postmenopausal women, as estrogen declines, RANK ligand expression increases.11 Elevated RANK ligand levels lead to increased osteoclast formation, function and survival.1,11,15 Greater osteoclast activity increases bone loss, weakens bone architecture, and can ultimately lead to fracture.1,5
We now understand the underlying biological mechanism of the increase in bone resorption that follows menopause.4,5,7,11,14
RANK ligand is a key link between reduced estrogen levels and osteoclast-mediated bone loss.1,11
References
1. Kostenuik PJ. Osteoprotegerin and RANKL regulate bone resorption, density, geometry and
strength. Curr Opin Pharmacol. 2005;5:618-625.
2. Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature.
2003;423:337-342.
3. US Department of Health and Human Services: Bone Health and Osteoporosis: A Report of the
Surgeon General. Washington DC.F 2004.
4. Riggs BL, Parfitt AM. Drugs used to treat osteoporosis: the critical need for a uniform
nomenclature based on their action on bone remodeling. J Bone Miner Res.2005;20:177-184.
5. Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects.
J Clin
Invest.
2005;115:3318-3325.
6. Seeman E, Delmas PD. Bone quality--the material and structural basis of bone strength and
fragility. N Engl J Med. 2006;354:2250-2261.
7. Chavassieux P, Seeman E, Delmas PD. Insights into material and structural basis of bone
fragility from diseases associated with fractures: how determinants of the biomechanical
properties of bone are compromised by disease. Endocr Rev. 2007;28:151-164.
8. Morgan EF, Barnes GL, Einhorn TA. The Bone Organ System: Form and Function. In: Marcus R,
Feldman D, Nelson DA, Rosen CJ, eds. Osteoporosis. 3rd ed. New York, NY: Elsevier
Academic Press; 2008:3-25.
9. Lacey DL, Tan HL, Lu J, et al. Osteoprotegerin ligand modulates murine osteoclast survival in
vitro and in vivo. Am J Pathol. 2000;157:435-448.
10. Dempster DW. Anatomy and Functions of the Adult Skeleton. In: Favus M, ed.
Primer on the
Metabolic Bone Diseases and Disorders of Mineral Metabolism.
6th ed. Washington, DC:
ASBMR; 2006:7-11.
11. Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, Riggs BL. Role of RANK ligand
in mediating increased bone resorption in early postmenopausal women. J Clin Invest.
2003;111:1221-1230.
12. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy.
Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785-795.
13. World Health Organization. Technical Report Series 921:
Prevention and Management of
Osteoporosis: Report of a WHO Scientific Group.
Geneva, Switzerland. 2003.
14. Hodgson SF, Watts NB, Bilezikian JP, et al. American Association of Clinical
Endocrinologists medical guidelines for clinical practice for the prevention and treatment of
postmenopausal osteoporosis: 2001 ed, with selected updates for 2003. Endocr Pract.
2003;9:544-564.
15. Kearns AE, Khosla S, Kostenuik PJ. Receptor activator of nuclear factor kappaB ligand and
osteoprotegerin regulation of bone remodeling in health and disease. Endocr Rev.
2008;29:155-192.
This transcript is provided for your convenience and is qualified by the full video in which the spoken content appears. Please review the full video along with the transcript.
The skeleton changes across the human life span. This is characterized predominantly by bone formation and growth throughout childhood, followed by a gradual loss of bone density that begins in early adulthood that can accelerate significantly in older adults.1,2
The density of bone is modulated by a group of cells including osteoclasts, which are multinucleated cells that resorb bone, and osteoblasts, which refill the resorption cavities created by osteoclasts.3
Osteoclasts anchor themselves to the surface of bone.3 This creates a microenvironment underneath the cell, which is referred to as the “sealed zone”.
Within this zone, the osteoclasts create an acidic environment that dissolves the bone’s mineral content.3 Once the mineral content of the bone has been dissolved, enzymes released from osteoclasts remove the remaining collagenous bone matrix to complete the process of resorption.3,4
Following resorption, osteoblasts move into the resorption space and start to produce and deposit organic matrix called osteoid. Osteoid, a substance made predominantly of collagen, forms a scaffold in which minerals, including calcium and phosphate, begin to crystallize.2,3,5 Some active osteoblasts become trapped within the matrix they secrete, and thereby become osteocytes.3
Other osteoblasts will undergo apoptosis or will revert back to lining cells which cover the surface of bone.3
This cycle of bone resorption and formation is referred to as remodeling. There is also a process where bone formation by osteoblasts occurs without prior bone resorption by osteoclasts; this results in an increase in bone mass and is referred to as bone modeling.6 Bone modeling promotes the growth of bones and is important for maintaining bone strength.6
Remodeling also plays an important role during bone growth by optimizing the growing structure.6
After the age of 30, most people experience a gradual loss in bone mass due to a relative decrease in the activity of osteoblasts compared with osteoclasts.1 However, there are many factors that impact the process of bone remodeling and influence the degree of bone loss we experience as we age. For example, medications, such as glucocorticoids, which can promote osteoclast activity and also reduce bone formation.7-9
Proper nutrition and physical activity can help strengthen bone.8,9 It is also believed that osteocytes form a complex network in bone that can sense any increased work load on the bone and respond by triggering the differentiation and activity of osteoblasts to increase bone density.9,10
Conversely, when bone experiences reduced loading conditions, such as during long term bed rest, resorption and remodeling increase to eliminate underloaded bone.9-11
Loss of bone mass reduces its strength and increases the risk of fracture.1 This highlights the importance of staying active, maintaining good nutrition throughout life, and being aware of personal risk factors associated with low bone density.8,9
References
1. Bergmann P, Body JJ, Boonen S, et al. Loading and skeletal development and maintenance.
J Osteoporos.
2010;2011:786752.
2. van der Linden JC, Homminga J, Verhaar JAN, et al. Mechanical consequences of bone loss in
cancellous bone.
J Bone Miner Res.
2001;16:457-465.
3. U.S. Department of Health and Human Services.
Bone Health and Osteoporosis: A Report of
the Surgeon General.
Rockville, MD: U.S. Department of Health and Human Services, Office
of the Surgeon General, 2004.
4. Jia D, O'Brien CA, Stewart SA, et al. Glucocorticoids act directly on osteoclasts to increase
their life span and reduce bone density. Endocrinology. 2006;147:5592-5599.
5. Moester MJC, Papapoulos SE, Löwik CWGM, et al. Sclerostin: current knowledge and future
perspectives. Calcif Tissue Int. 2007;87:99-107.
6. Nather A, Ong HJC, Aziz Z. Structure of Bone. In: Nather A.
Bone Grafts and Bone
Substitutes: Basic Science and Clinical Applications.
World Scientific Publishing
Company; 2005:3-18.
7. Prentice A, Schoenmakers I, Laskey MA, et al. Nutrition and bone growth and development.
Proc Nutr Soc. 2006;65:348-360.
8. Raggatt LJ, Partridge NC. Cellular and molecular mechanisms of bone remodeling.
J Biol
Chem.
2010;285:25103-25108.
9. Saftig P, Hunziker E, Wehmeyer O, et al. Impaired osteoclastic bone resorption leads to
osteopetrosis in cathepsin-K-deficient mice. Proc Natl Acad Sci U S A.
1998;95:13453-13458.
10. Seeman E. Osteocytes--martyrs for integrity of bone strength. Osteoporos Int.
2006;17:1443-1448.
11. Simão AMS, Yadav MC, Ciancaglini P, et al. Proteoliposomes as matrix vesicles' biomimetics
to study the initiation of skeletal mineralization. Braz J Med Biol Res. 2010;43:234-241.
12. Zerwekh JE, Ruml LA, Gottschalk F, et al. The effects of twelve weeks of bed rest on bone
histology, biochemical markers of bone turnover, and calcium homeostasis in eleven normal
subjects. J Bone Miner Res. 1998;13:1594-1601.
This transcript is provided for your convenience and is qualified by the full video in which the spoken content appears. Please review the full video along with the transcript.
The human skeleton gives the body its shape and provides physical support for the system contained within.1 It also forms part of the musculoskeletal system that enables us to move.1 The structure of bone is optimized so that it is strong but relatively light weight.The interior of bone is composed of bone marrow.2 It is surrounded by two major types of bone tissue; cortical bone, or the hard outer shell of bone, and trabecular bone, the spongy-looking center.2 The amount of each type of tissue in bone is dependent on the function of that bone.2
The basic unit of cortical or compact bone is the osteon.2 It is composed of successive concentric lamellae.2 This structure contributes to bone strength by resisting bending.2
Cells called osteocytes are distributed within the concentric lamellae.2 Osteocytes form a complex network3 that is thought to be important in maintaining the viability and structural integrity of bone.4
At the center of the osteon is the Haversian canal. These canals contain blood vessels and nerves.2 The blood vessels within bone facilitate the exchange between osteocytes and the blood.2
Trabecular bone is present in the interior of some bones and resists compression.2 Osteocytes are also contained with its structure and again play an important role in sensing local changes in strain.5 Trabeculae are covered in a layer of flattened lining cells that are thought to be involved in the dynamic process by which bone is formed and broken down.2
Bone marrow is found within the interior of bones. The surrounding trabeculae and vascular network provide structural support, nutrition and a waste removal system for the heterogeneous group of cells found within this space.6 Bone marrow is a site for haemopoiesis, the process by which the cellular components of blood are formed.6
Bone is a dynamic tissue that is continually being built, broken down and rebuilt in a process called bone remodeling.1
Bone tissue is broken down and resorbed by multinucleated cells known as osteoclasts.1 These cells are derived from monocytes which originate within bone marrow.6 Osteoclasts play an important role in liberating minerals and other molecules stored within the bone matrix.7,8
Bone tissue serves as a repository for vital minerals, including calcium phosphate,7 and various biologically active molecules, such as growth factors.8 The release of calcium from the bone can play a role in maintaining its homeostasis within the body.7
The cells responsible for building new bone tissue are known as osteoblasts.1 Osteoblasts are thought to be derived from cells found to be associated with blood vessels.2 Once active, they start to produce the organic component of bone osteoid, which is predominantly made of collagen.1
Minerals start to crystallize around the collagen scaffold to form hydroxyapatite, the major inorganic constituent of bone, which contains calcium phosphate.3,4 Bone mineral density (or BMD) can be used to estimate the strength of bone and to assess the risk of fracture.5
As osteoblasts form new bone tissue, many become embedded within the matrix and differentiate into osteocytes.1
The structure, composition, and cellular processes that occur within bone allow it to simultaneously serve as a calcium reservoir,7 while providing structural support for the vital organs and for locomotion.9
References
1. Adachi T, Kameo Y, Hojo M. Trabecular bone remodelling simulation considering osteocytic
response to fluid-induced shear stress. Phil Trans R Soc A 2010;368:2669-2682.
2. Doherty MJ, Ashton BA, Walsh S, et al. Vascular pericytes express osteogenic potential in
vitro and in vivo. J Bone Miner Res. 1998;13:828-838.
3. Hanssens L, Reginster JY. Relevance of bone mineral density, bone quality and falls in
reduction of vertebral and non-vertebral fractures. J Musculoskelet Neuron Interact.
2003;3:189-193.
4. Komarova SV. Mathematical model of paracrine interactions between osteoclasts and osteoblasts
predicts anabolic action of parathyroid hormone on bone. Endocrinology.
2005;146:3589-3595.
5. Nather A, Ong HJC, Aziz Z. Structure of Bone. In: Nather A.
Bone Grafts and Bone
Substitutes: Basic Science and Clinical Applications.
World Scientific Publishing
Company; 2005:3-18.
6. Raggatt LJ, Partridge NC. Cellular and molecular mechanisms of bone remodeling.
J Biol
Chem
. 2010;285:25103-25108.
7. Simão AMS, Yadav MC, Ciancaglini P, et al. Proteoliposomes as matrix vesicles' biomimetics to
study the initiation of skeletal mineralization. Braz J Med Biol Res. 2010;43:234-241.
8. Tinkler SMB, Linder JE, Williams DM, et al. Formation of osteoclasts from blood monocytes
during 1 alpha-OH Vit D-stimulated bone resorption in mice. J Anat. 1981;133:389-396.
9. Vashishth D, Verborgt O, Divine G, et al. Decline in osteocyte lacunar density in human
cortical bone is associated with accumulation of microcracks with age. Bone.
2000;26:375-380.
10. Watkins J. The Skeleton. In: Watkins J.
Structure and Function of the Musculoskeletal System.
Human Kinetics Publishers, Inc; 2010:21-58.
11. Wilkins BS. Histology of normal haemopoiesis: bone marrow histology I. J Clin Pathol.
1992;45:645-649.
12. Yin JJ, Pollock CB, Kelly K. Mechanisms of cancer metastasis to the bone.
Cell
Res.
2005;15:57-62.